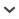A survey of spiders from Moroccan caves is provided (Arachnida: Araneae), collected between July 2012 and October 2022. All material originates from 38 caves in various mountainous areas located in the northern part of the country. In total 24 species from eight families have been recorded. A new cave-dwelling spider species from the Moroccan High Atlas, a representative of the Dysdera crocata-complex, is described: Dysdera agadirensis Lecigne spec. nov. Another new cave dwelling species, Centromerus caecus Lecigne spec. nov., the first known eyeless representative of Centromerus in North Africa, is described and illustrated as well. Two further agelenid species spiders are also newly described: Eratigena talassemtane Lecigne & Bosmans spec. nov. and Textrix maroccana Lecigne spec. nov. The male of Lepthyphantes leknizii Barrientos, 2020 was discovered and is described here for the first time. Two other records represent new species records for the fauna of Morocco: Amaurobius barbarus Simon, 1911 and Scotargus pilosus Simon, 1913. Lepthyphantes pieltaini Machado, 1940 is a poorly known species; we provide a redescription based on several specimens of both sexes. The findings of several other species already known from Morocco are noteworthy taxa (either endemic or recently described species, but also species with a poorly known taxonomy, ecology or distribution) and hence represent important records for the local fauna: Lepthyphantes aelleni Denis, 1957, Lepthyphantes fadriquei Barrientos, 2020, Lepthyphantes maurusius Brignoli, 1978, Lepthyphantes taza Tanasevitch, 2014, Maghreba aurouxi (Barrientos, 2019), Palliduphantes banderolatus Barrientos, 2020 and Tama edwardsi (Lucas, 1846). Drawings and photographs of most of these species are provided.
Eine Untersuchung von Spinnen aus marokkanischen Höhlen wird präsentiert. Das Material stammt aus 38 Höhlen in verschiedenen gebirgigen Regionen aus dem nördlichen Teil des Landes. Insgesamt wurden 24 Arten aus 8 Familien festgestellt. Eine neue höhlenbewohnende Spinne wird aus dem Marokkanischen Hohen Atlas beschrieben: Dysdera agadirensis Lecigne spec. nov. Eine weitere höhlenbewohnende Art, Centromerus caecus Lecigne spec. nov., die erste bekannte augenlose Vertreterin der Gattung Centromerus in Nordafrika, wird ebenfalls beschrieben und abgebildet. Zwei weitere Arten der Agelenidae werden ebenfalls neu beschrieben: Eratigena talassemtane Lecigne & Bosmans spec. nov. and Textrix maroccana Lecigne spec. nov. Das Männchen von Lepthyphantes leknizii Barrientos, 2020 wurde entdeckt und wird hier zum ersten Mal beschrieben. Zwei Nachweise stellen die erste Artnachweise für die Fauna von Marokko dar: Amaurobius barbarus Simon, 1911 und Scotargus pilosus Simon, 1913. Lepthyphantes pieltaini Machado, 1940 ist eine wenig bekannte Art; eine Wiederbeschreibung basierend auf zahlreichen Individuen beider Geschlechter wird präsentiert. Die Funde zahlreicher weiterer Arten, die bereits aus Marokko bekannt sind, stellen nennenswerte Taxa dar (endemische oder kürzlich beschriebene Art, aber auch Arten mit einer wenig bekannten oder beschriebenen Taxononomie, Ökologie oder Verbreitung) und sind daher wichtige Nachweise für die lokale Fauna: Lepthyphantes aelleni Denis, 1957, Lepthyphantes fadriquei Barrientos, 2020, Lepthyphantes maurusius Brignoli, 1978, Lepthyphantes taza Tanasevitch, 2014, Maghreba aurouxi (Barrientos, 2019), Palliduphantes banderolatus Barrientos, 2020 and Tama edwardsi (Lucas, 1846). Zeichnungen und Bilder von den meisten dieser Arten werden präsentiert.
The Moroccan spider fauna currently numbers only 487 species (Nentwig et al. 2023). Given the location of the country in the Mediterranean basin, a global biodiversity hotspot, this low number explains why many spider species (including species new to science) are added to the national checklist every year (e.g. Bayer et al. 2017, Benhalima & Bosmans 2020, Massa & Ribera 2021). The poor knowledge of the Moroccan spider fauna is especially reflected in cave environments, a traditionally understudied habitat that yielded several species new to science over the last years (e.g. Tanasevitch 2014, Barrientos et al. 2020, Huber 2021, 2022). The present paper is the first in a series of contributions aiming to improve the knowledge of the taxonomy, distribution and ecology of cave-dwelling spiders of Morocco. Here we present the results of studies carried out within the context of several scientific projects and expeditions, all in collaboration with the Natural History Museum of Marrakech, University Cadi Ayyad:
– A thesis conducted by the second author on cave macroinvertebrates of Morocco
– A French-Moroccan speleological expedition in the Talassemtane National Park, with the goal of improving the knowledge of this massif that includes the deepest chasm in Morocco. One of the main parts of the project was the exploration and the description of the topography of more than 21 km of underground systems
– Second edition of the Agadir scientific internship, in collaboration between the French Speleology Federation (FFS) – Scientific Commission and the Moroccan National Speleology Federation
– Moroccan-Spanish expedition: in collaboration between the Associació Catalana de Bioespeleologia (Biospel) and the Museum of Natural Sciences of Barcelona
– Moroccan-Brazilian Expedition: in collaboration with the Center of Studies on Subterranean Biology, Federal University of Lavras with the aim of surveying the fauna of some caves in several regions of Morocco (Agadir, Errachidia, Taza, Safi and Echemmaia).
In this paper, we also present the results of several surveys carried out intermittently between July 2012 and October 2022. A total of 38 caves or groups of natural cavities in six main locations (e.g. the two-part cave system known as Imi Ougoug in Agadir area) were prospected (Fig. 1). Figure 2 shows the main caves surveyed in several mountain ranges, mainly in the Atlas Mountains, but also in the North (Rif Mountains) and North-East of the country (beginnings of the Tell Mountains). Information on the caves surveyed is detailed in Tab. 1.
Material and methods
Specimens were collected by hand and preserved in situ in alcohol (96% or 75% ethanol) for identification purposes. All information on the observations collected during the surveys is gathered in two private databases completed and updated by the second and third authors. Species were examined by using a Nikon SMZ800N and a Nikon SMZ1270 stereo microscope. Most of the photographs of genitalia were taken under an Olympus CH-2 microscope.
Somatic measurements were made with a scaled eyepiece in the stereo microscope and are expressed in mm. Measurements of the legs are taken from the dorsal side. The length of the chelicerae is given from the edge of the carapace. Geographic coordinates are presented in the WGS 84 system.
For identification we relied on several publications and reference websites: Simon (1909, 1911), Machado (1940), Denis (1954, 1961), de Blauwe (1980), Bosmans (1986, 2006a, 2006b, 2021), Hormiga & Ribera (1990), Rheims & Brescovit (2004), Benhadi-Marín et al. (2013), Tanasevitch (2014), Barrientos et al. (2019, 2020), Breitling (2020), Huber (2022), Nentwig et al. (2023), Oger (2023), etc. The taxonomic status follows the WSC (2023). Unless otherwise specified, samples are conserved in the private collection of the first author; drawings were made by the first author. The terminology of genital structures in Micronetinae follows that of Saaristo & Tanasevitch (1996).
For the purpose of this study, we use the traditional speleobiological nomenclature (Sket 2008) to indicate ecological distributions of species: a troglobiont is strongly bound to hypogean habitats; a troglophile is able to maintain hypogean populations, but relies on epigean habitats for some biological functions; a trogloxene occurs only sporadically in a hypogean habitat and is unable to establish stable subterranean populations.
Abbreviations
AP – apical part of paracymbium; C – conductor; CD – copulatory duct; E – embolus; Fe – femur; FGL – Fickert's gland; j – juvenile; LC – lamella characteristica; MA – median apophysis; MP – middle part of paracymbium; MPS – median part of scape; Mt – metatarsus; PL – prosoma length; PLE – posterior lateral eyes; PME – posterior median eyes; PMP – posterior median plate; PP – proximal part of paracymbium; PPO – posterior pocket of paracymbium; PS – proscape (proximal part of scape); PW – prosoma width; RTA – retrolateral tibial apophysis; SA – suprategular apophysis; Sc – scape; SMF – Senckenberg Museum Frankfurt; Sp – spermatheca; St – stretcher; TA – terminal apophysis; Te – tegulum; Ti – tibia; Tm I – relative position of trichobothrium on metatarsus I
Results
In total, 904 specimens belonging to 24 species and 9 families were examined for this study. The complete list of records is presented in the Appendix (Tab. S1).
Descriptions of species new to science
Centromerus Dahl, 1886 (Linyphiidae)
Centromerus caecus Lecigne spec. nov. (Fig. 3a-g)
By the structure of the epigyne/vulva, it seems to be closely related to Centromerus dacicus Dumitrescu & Georgescu, 1980, but also to several Centromerus species of the europaeuscomplex from the caves of the Balkan Peninsula (Deltshev & Ćurčić 1997, 2002). In general, most troglobiont species have a limited range. Hence, it is reasonable to assume that the specimens found in this cave of the Talassemtane National Park belong to a new species. The terminology of genital structures follows that of Bosmans (1986), based on Merret (1963) and Millidge (1977).
Type material. Holotype: 1 ♀, MOROCCO: Chefchaouen (province), Sef Lahmer V cave (SLV) (35.10213°N, -5.15950°W, 1484 m a.s.l.), hand collecting, 25. Sep. 2019, leg. S. Moutaouakil.
Remark. Opisthosoma and epigyne detached. Paratype: 2 ♀♀, MOROCCO: Same as holotype. Type material will be deposited in the SMF.
Other material examined. MOROCCO: Same as type material, 1 j, 1 ♀ (coll. P. Oger).
Etymology. The name relates to the Latin adjective ‘caecus’ and refers to the eyelessness of the new species.
Diagnosis. By its total absence of eyes, the new species differs from all the others currently known from the Maghreb (Bosmans 1986, 2006b, WSC 2023). Besides, due to its small size (1.1–1.3 mm), the shape and the poor chitinisation of its epigyne, C. caecus spec. nov. differs from most other North African species i.e. Centromerus desmeti Bosmans, 1986, Centromerus paradoxus (Simon, 1884), Centromerus prudens (O. Pickard-Cambridge, 1873) and Centromerus sinuatus Bosmans, 1986. In its pale coloration, the new species most resembles Centromerus cinctus (Simon, 1884), but differs in the orientation of the anterior part of the spermathecae: directed inwards for the new species (cf. Fig. 3g) and outwards for C. cinctus. This feature also distinguishes C. caecus spec. nov. from Centromerus phoceorum Simon, 1929. Finally, the new species can be distinguished from Centromerus succinus (Simon, 1884) by the shape of the copulatory ducts: from the copulatory openings, inclined outwards, without extending anteriorly beyond the spermathecae for C. caecus spec. nov.; from the copulatory openings, almost straight, then forming a large anterior loop extending distinctly beyond the spermathecae for C. succinus.
Description. Female. Measurements (n = 4) (min.–max. (average): total length 1.12–1.32 (1.23); PL 0.52–0.60 (0.57), PW 0.43–0.49 (0.47), PL/PW 1.21–1.22 (1.21); sternum length 0.32–0.43 (036), sternum width 0.33–0.38 (0.36); chelicerae 0.28 long.
Colour (based on specimens in alcohol): prosoma, legs and sternum very pale yellow, chelicerae only slightly darker; opisthosoma uniformly pale grey.
Eyes: completely absent (Fig. 3a, c).
Chelicerae: three promarginal teeth, four retromarginal minute teeth, arranged in group, the furthest from the fang joint being slightly larger.
Legs (min.–max.): chaetotaxy 2221; Fe I, one prolateral spine in the distal quarter; Ti I, position of first spine 0.20–0.22 (0.21), position of second spine 0.76–0.78 (0.77); Mt I, one spine in position 0.51, position of trichobothrium 0.26; Mt II, one spine in position 0.52; trichobothrium on Mt IV absent.
Epigyne: poorly chitinised. Wide genital fossa, anteriorly provided with a folded scape whose basal part occupies almost the entire width of the fossa (Fig. 3d). The distal part of the scape narrows strongly (Fig. 3f). The lateral parts of the copulatory ducts are visible in transparency on either side of the distal part of the scape (Fig. 3d, f).
Vulva: spermathecae inclined inwards, rounded anteriorly and posteriorly elongated (Fig. 3g). Copulatory ducts posteriorly inclined outwards by about 45° (Fig. 6e, g) without extending anteriorly beyond the spermathecae.
Male. Unknown.
Comment. Currently the only eyeless species of the genus in North Africa.
Distribution and habitat. Only known from the Sef Lahmer V cave (Chefchaouen, Morocco) in the Western Rif (Fig. 1).
Dysdera Latreille, 1804 (Dysderidae)
Dysdera agadirensis spec. nov. Lecigne (Fig. 4a-f)
The specimens from Agadir clearly belong to the Dysdera crocata-complex as defined by Deeleman-Reinhold & Deeleman (1988): characterized by a wide and flat prosoma, straight anterior margins, massive chelicerae and femora IV armed with spines.
The terminology of genital structures follows that of Deeleman-Reinhold & Deeleman (1988).
Type material. Holotype: 1 ♀, MOROCCO: region of Souss-Massa, prefecture of Agadir Ida-Outanane, Aqsri, village of Tizgui, Imi Ougoug cave system, “Grotte des Chauves-souris” area (GCS) (30.61289°N, 9.46712°W, 780 m a.s.l.), on walls of the cave near the siphon (very wet area, presence of a lot of guano), hand collecting, 28. Oct. 2019, leg. B. Lips (will be deposited in the SMF). Remark: Opisthosoma and epigyne detached. Paratype: 1 ♀, MOROCCO: Same location as holotype.
Other material examined. MOROCCO: Same as type material, 1 j, hand collecting, 28. Oct. 2019, leg. B. Lips. Same as type material, 1 ♀, hand collecting, 30. Oct. 2019, leg. J. Lips.
Etymology. The name of the species refers to “Agadir”, the prefecture of the region of Morocco where it was discovered.
Diagnosis. The shape of the dorsal and ventral arches, spermatheca and transversal bar, but especially the anterior diverticulum enables D. agadirensis spec. nov. to be distinguished from other representatives of the species complex with available information on the female genitalia (but see Comment below). The anterior margin of the dorsal arch of the diverticulum is more rounded than in D. crocata C. L. Koch, 1838 and D. pharaonis Simon, 1907. Dysdera subnubila Simon, 1907 (known especially from the eastern Maghreb) has a rounded dorsal arch, similar to D. agadirensis spec. nov., but the orientation of the spermatheca is very different: directed forwards for the latter, backwards for D. subnubila. Unlike in D. agadirensis spec. nov., the dorsal arch of D. longimandibularis Nosek, 1905 is wider than the spermatheca, short and the posterior ends clearly bifid. Dysdera presai Ferrández, 1984 (known only from Spain) shows similarities in terms of chaetotaxy, but the vulvae of the two species are different: outer margins of spermatheca slightly forward in D. agadirensis spec. nov., straight in D. presai; anterior part of the dorsal arch of D. agadirensis spec. nov. narrower; outer margins of the transversal bar point slightly backward in D. presai, but are straight in D. agadirensis spec. nov. The anterior margin of the spermatheca is clearly concave in D. atlantica, but slightly convex in D. agadirensis spec. nov; the dorsal arch of D. atlantica is much longer (see Comment).
Description. Female. Medium-sized species, all femora armed with spines.
Measurements: female (n = 3) (min.–max. (average)): total length 9.40–11.10 (10.13); PL 4.40–4.65 (4.55), PW 3.40–3.60 (3.48), ratio PL/PW = 1.28–1.35 (1.31); chelicerae 2.00–2.07 (2.02) long.
Colour (Fig. 4a-b, d): prosoma reddish orange, edged by a thin black line more marked anteriorly; chelicerae reddish orange; legs orange, hind ones lighter; sternum orange brown; opisthosoma pale yellow.
Prosoma (Fig. 4b): flat, with very fine granulation. Anterior eyes separated by one time their diameter. Height of clypeus: 1.1 times the diameter of the anterior eyes. Chelicerae massive, projected, margin with three teeth, the middle one the smallest; basal part with a smooth keel (Fig. 4c).
Legs: chaetotaxy (in brackets: less frequent pattern for spines number) (Fig. 4b): Fe I, 2–3 prolateral-apical (last quarter) spines; Fe II, 1 prolateral-apical (last quarter) spine; Fe III, 2–3 prolateral spines aligned (or 1 anterior – half or last quarter – spine); Fe IV, 4-5 (6) dorso-basal spines.
Vulva (Fig. 4e-f): dorsal arch of diverticulum 1.6 times wider (without posterior ends) than long, evenly rounded, lateral sides straight, posterior extremities directed outwards, posterior cavity regularly convex; spermatheca only slightly wider than dorsal arch of diverticulum (in its median part), outer margins directed slightly forwards; transverse bar sub-rectilinear, 2 times wider than spermatheca, extremities rounded.
Male. Unknown.
Comment. Dysdera atlantica is one of the 110 species of Arachnida recorded by M. de la Escalera during his journey to Morocco in 1907, material subsequently studied by Eugène Simon (1909). The specimens of Dysdera agadirensis spec. nov correspond to Simon's description of D. atlantica (chetotaxy, especially of the femora, and few other morphological features i.e. colouration, eye area and clypeus). Dysdera atlantica is also the only species among those mentioned by Denis (1961, tab. p. 150) from Morocco that corresponds in terms of chaetotaxy. However, Simon (1909) does not provide any information whatsoever on the internal genital structure of the female. Nonetheless, a comparison of the specimens of Dysdera agadirensis spec. nov. with a photo (kindly provided by Robert Bosmans) of the vulva of a specimen from his collection revealed that they did not match D. atlantica. Also, the vulvae of Dysdera mauritanica Simon, 1909, Dysdera ravida Simon, 1909 or Dysdera snassenica Simon, 1911 do not match (Bosmans, pers. comm.).
This case highlights the fact that chaetotaxy does not, on its own, enable a confirmation of a species of the genus Dysdera, at least for the crocata-complex. It must necessarily be completed by an analysis of the genitalia. Denis (1961) already pointed out the “... variations individuelles susceptibles d'affecter la chétotaxie ...” and the “instabilité relative ...” of this feature within this genus, considered at the time especially for females in the absence of a study of the vulva.
The ecology of the new species remains poorly known but, even if it does not show any adaptation to subterranean life (features which are not systematic for all troglobiont species), all the specimens were collected very close to the siphon (see the map of Imi Ougoug cave system, Lecigne et al. 2020, fig. 3), which is far inside the cave. The Imi Ougoug cave system has already been explored several times. The species was never observed in the vicinity of the entrances, either inside or outside (see also above for expeditions of M. de la Escalera) or even at other locations in North Africa (e.g. numerous surveys of the Maghreb by R. Bosmans, pers. comm.). All these elements consequently lead us to retain the troglobiont character for this new species.
Distribution and habitat. Only known from the type locality (Tizgui, Agadir, Morocco) (Fig. 2); in one cave system (Imi Ougoug, “Grotte des Chauves-souris”) in the Western High Atlas (Fig. 1).
Fig. 3.
Centromerus caecus sp. nov., female. a. dorsal view; b. ventral view; c. frontal view; d. epigyne; e, g. vulva, dorsal view; f. vulva, ventral view (photos: P. Oger). Scale line (f, g) = 0.05 mm. Abbreviations: CD – copulatory duct; CO – copulatory opening; Sc – scape; Sp – spermatheca

Eratigena Bolzern, Burckhardt & Hänggi, 2013 (Agelenidae)
Eratigena talassemtane spec. nov. Lecigne & Bosmans (Fig. 5a-j)
Type material. Holotype: 1 ♂, MOROCCO: Chefchaouen-Bab Taza, Talassemtane National Park, Toghobeit cave (TOG) (35.08960°N, -5.13741°W, 1710 m a.s.l.), hand collecting, 10. Aug. 2018, leg. S. Moutaouakil. Remark: right legs I to III missing; right leg IV detached, as well as left pedipalp. Type material will be deposited in the SMF.
Etymology. The name of the species refers to “Talassemtane”, the name of the National Park where it was discovered.
Diagnosis. By the presence of six teeth on the cheliceral retromargin, this species is classified in Eratigena. It is closely related to E. fuesslini (Pavesi, 1873) from Europe, but can be separated from the latter by the shape of the dorsal branch of the RTA, having a triangular and robust ventral part and a dorsal part shaped like a small tooth. In E. fuesslini, the dorsal branch of the RTA is broad, distally truncated, and not having a dorsal part. The female remains unknown.
Description. Male. Measurements (n = 1): total length 8.9; PL 4.8, PW 3.3.
Colour (based on specimens in alcohol): prosoma greyish brown, with wide pale median and marginal bands; chelicerae brown; sternum yellowish with distinct pale median band tape in the rear half; legs pale yellowish, not annulated, metatarsi and tarsi darkened; opisthosoma dorsally greyish brown with 2 lines of lighter spots extending posteriorly by chevrons.
Chelicerae: Cheliceral promargin with 3 teeth, the middle one the largest, the proximal one the smallest; cheliceral retromargin with 6 teeth, the four distal ones subequal and conical, the two proximal ones of decreasing size.
Metatarsi I with long hairs.
Male palp (Fig. 5b-j): RTA with two branches, the ventral one small, conical and translucid, pointing in anterior direction (Fig. 5d, j), the dorsal one strongly sclerotized, triangular, robust, with broad base, the dorsal part a small sharp tooth pointing backward (Fig. 5d, j); median apophysis partly membranous and partly with a distal accent-shaped sclerite, weakly sclerotized, prolaterally in the shape of a stretched lamella with almost parallel edges (Fig. 5g-h); embolus filiform, originating at a 10 o'clock position, tip at a 5 o'clock position; conductor terminally bifid, strongly sclerotized, unequal, the prolateral branch larger and slightly twisted (Fig. 5h, PBC)
Female. Unknown.
Distribution. Only known from Toghobeit cave, the type locality, located in the Talassemtane National Park. Toghobeit cave is the deepest known cave in Morocco.
Fig. 4.
Dysdera agadirensis spec. nov., female. a-b. dorsal view; c. chelicerae, ventral view; d. ventral view; e-f. vulva, dorsal view (Photos a: J. Lips; b-e: P. Oger). Abbreviations: DA – dorsal arch of anterior diverticulum; Sp – spermatheca; TB – transversal bar or dorsal arch of posterior diverticulum

Fig. 5.
Eratigena talassemtane sp. nov., male holotype. a. dorsal view; b. palp, dorsal view; c. idem, apical part of the conductor, retrolateral view; d. palp, RTA; e-f. palp, prolateal view; g-h. idem, ventral view; i-j. idem, retrolateral view (Photos a: S. Lecigne; b-e, g, i: P. Oger). Abbreviations: C – conductor; E–embolus; MA– median apophysis; PBC– prolateral branch of the conductor; RTA d, l, v– retrolateral tibial apophysis, respectively dorsal, lateral, and ventral branches

Lepthyphantes Menge, 1866 (Linyphiidae)
Lepthyphantes leknizii Barrientos, 2020 (Figs 6, 7, 28)
Barrientos et al. (2020): p. 13, fig. 8D.
Material examined. MOROCCO: Taza (province), Ghar Admam (same cave as holotype, according to collector Floren Fadrique; Barrientos, pers. comm.), 1 ♂, hand collecting, 1. Nov. 2018, leg. J. Lips (will be deposited in the SMF).
Diagnosis. The species is characterized by long legs, the pale colouration as well as by the absence of eyes in both sexes. The male described herein can easily be recognized by the very distinctive shape of the lamella characteristica and the paracymbium.
Description. Male. Measurements (n = 1): total length 2.25; PL 1.24, PW 1.00; sternum 0.65 long, 0.68 wide; chelicerae 0.47 long femora, tibiae and metatarsi I 1.97 long; metatarsi IV 1.83 long.
Colour (from specimen in alcohol): Carapace, chelicerae, sternum and legs yellowish orange, opisthosoma pale grey, nearly whitish, dorsal pattern absent. Eyes are completely absent (Fig. 6c). Chelicerae bear three promarginal teeth, the middle one the largest; two retromarginal minute teeth. Legs long, Fe I, Ti I and Mt I 1.6 times as long as prosoma; chaetotaxy 2222; tibiae I, position of first spine 0.30, position of second spine 0.72; one spine on Mt I in position 0.25; one spine on Mt IV in position 0.31; position of trichobothrium on Mt I 0.128; trichobothrium on Mt IV absent. Prosoma with several long setae in the cephalic region (Fig. 7b). Opisthosoma with some long setae.
Pedipalp (Figs 6a-c, 7d-g): Paracymbium wide, proximal part with seven scattered spines and a protrusion on the front edge, middle part with a strong notch bordered by two teeth, the longer and stouter one pointed outwards (Fig. 6a), distal part slender and terminally slightly enlarged; suprategular apophysis pointed; lamella characteristica broad, markedly widened distally (Fig. 6b, LC); terminal apophyses broad at its base then tapered, apically curved and directed outwards (Fig. 6b, TA).
Distribution and habitat. Only known from the type locality (Taza, Morocco) (Figs 1, 28) in one cave (Ghar Admam, Fig. 2h) between the Rif and the Middle Atlas.
Comments. The examined male specimen was determined as L. leknizii based on its occurrence at the type locality. Since the taxonomy of the species of Micronetinae Hull, 1920 has not been clarified and stabilized, including species from the Mediterranean region, this species was provisionally described in the genus Lepthyphantes s. lat. by Barrientos et al. (2020). It does not belong to the genus Lepthyphantes Menge, 1866 sensu Saaristo & Tanasevitch (1996) as it does not fit the diagnosis for the genus presented by the latter authors (e.g. embolus of the species not sickle-shaped). According to the conformation of the genitalia, the species actually belongs to the afer-complex of Lepthyphantes s. lat. (Saaristo & Tanasevitch 1993). The female is closely related to L. afer (Barrientos et al. 2020). It shows typical troglobiont features, i.e. no eyes, pale leg and body coloration as well as long legs.
Fig. 6.
Lepthyphantesleknizii, male, pedipalp. a. retrolateral view; b. idem, ventral view; c. idem, prolateral view. Abbreviations: AP – apical part of paracymbium; FGL – Fickert's gland; LC – lamella characteristica; MP – middle part of paracymbium; PP – proximal part of paracymbium; SA – suprategular apophysis; TA – terminal apophysis

Fig. 7.
Lepthyphantes leknizii, male. a-b. dorsal view; c. frontal view; d. pedipalp, dorsal view; e. idem, ventro-retrolateral view; f. idem, ventral view; g. idem, ventro-prolateral view (photos: P. Oger)

Fig. 8.
Lepthyphantes pieltaini. a. male, dorsal view; b. male pedipalp, ventro-retrolateral view; c. idem, ventral view; d. idem, prolateral view; e. idem, ventro-prolateral view; f-g. idem, lamella characteristica, middle and distal parts of paracymbium; h. female, dorsal view; i. idem, ventral view; j-k. epigyne, ventral view; l. idem, lateral view (photos: P. Oger). Abbreviations: AP – apical part of paracymbium; APO – anterior pocket of paracymbium; FGL – Fickert's gland; LC – lamella characteristica; MP – middle part of paracymbium; MPS – median part of scape; PMP – posterior median plate; PP – proximal part of paracymbium; PPO – posterior pocket of paracymbium; PS – proscape (proximal part of scape); SA – suprategular apophysis; St – stretcher; TA – terminal apophysis; Te – tegulum

Fig. 9.
Textrix maroccana sp. nov., male holotype. a. dorsal view; b. palp, dorsal view; c. Idem, RTA; d. idem, ventro-retrolateral view; e-f. idem, prolateral view (e, arrow: prolateral extension of the conductor); g-h. idem, ventral view; i-j. idem, retrolateral view; k. idem, RTA (photos: P. Oger). Scale lines: f, h, j = 0.5 mm; k = 0.2 mm. Abbreviations: C – conductor; E– embolus; RTA – retrolateral tibial apophysis

Fig. 10.
Lycosoides parva, male, pedipalp. a. prolateral view; b. ventral view; c. retrolateral view.

Fig. 11.
Amaurobius barbarus, male. a. dorsal view; b. tibial apophyses, retrolateral view; c. pedipalp, retrolateral view; d. idem, ventral view; e. idem, prolateral view (photos: P. Oger)

Lepthyphantes pieltaini Machado, 1940 (Figs 8a-l, 29) Machado (1940): p. 516, figs 2-3, 5-6 (fm).
New records. MOROCCO: Chefchaouen (province), Aframanou cave, 2 ♀♀, 2 jj, hand collecting, 8. Aug. 2018, leg. S. Moutaouakil. Idem, Ain d'Anou cave, 2 ♀♀, hand collecting, 23. Aug. 2018, leg. J.-P. Degletagne. Idem, 3 ♀♀, leg. S. Moutaouakil. Idem, 1 ♀, 30. Sep. 2019, leg. S. Moutaouakil. Idem, Aouta El Gazdir cave, 1 ♂, hand collecting, 19. Jul. 2010, leg. M. Chassier. Idem, 3 ♀♀, 1 j, 22. Jul. 2012, leg. M. Chassier. Idem, 1 ♀, 25. Jul. 2012, leg. M. Chassier. Idem, 1 ♀, 7. Aug. 2018, leg. S. Moutaouakil. Idem, Fouk Magou cave, 1 ♂, 34 jj, hand collecting, 26. Sep. 2019, leg. S. Moutaouakil. Idem, Gazdir 2 cave, 1 ♀, hand collecting, 19. Jul. 2010, leg. M. Chassier. Idem, 1 ♂, 5 ♀♀, hand collecting, 22. Aug. 2018, leg. S. Moutaouakil. Idem, Ghar Gharnaji cave (Kehf de Ouad N'Ghir), 4 ♀♀, 14 jj, hand collecting, 29. Sep. 2019, leg. S. Moutaouakil. Idem, Kehf del Khashab Alqayqab cave, 1 ♂, hand collecting, 21. Aug. 2018, leg. S. Moutaouakil. Idem, Kehf N'Ghar cave, 1 ♀, hand collecting, 14. Aug. 2018, leg. S. Moutaouakil. Idem, Toghobeit cave, 8 ♀♀, 1 j, hand collecting, 10. Aug. 2018, leg. S. Moutaouakil.
Type material. Missing (see Ribera 1983, Bosmans 2006a).
Diagnosis. The male of L. pieltaini closely resembles L. longihamatus in the bifid supra-tegular apophysis and in the shape of the lamella characteristica and the paracymbium (see also Comment). However, it differs from the latter by the presence of a large, rounded extension on the middle part of the paracymbium (Fig. 7c) and by a much more discreet tooth in the posterior pocket of the paracymbium. The male of L. pieltaini can be distinguished from L. bidentatus mainly by the shape of the distal part of the lamella characteristica: distinctly widened for L. pieltaini with two rounded lobes visible in retrolateral view; with high front margin for L. bidentatus (Hormiga & Ribera 1990: 43, fig. 13, “l.c.”). In size (as wide as long) and shape of its proscape (triangular), the epigyne of L. pieltaini resembles no other species of the afer-complex.
Description. Male. Measurements (n = 2) (min.–max. (average)): total length 2.7–3.2 (2.95); PL 1.23–1.45 (1.34), PW 1.03–1.15 (1.09), PL/PW 1.20–1.26 (1.23); sternum 0.70–0.75 (0.73) long, 0.68–0.75 (0.72) wide; chelicerae 0.50–0.60 (0.55) long; Fe I 2.28 long, Ti I 2.37–2.43 (2.40) long, Mt I 2.30–2.47 (2.38) long.
Colour (based on specimens in alcohol): Carapace, chelicerae and legs orange-brown, the margin of the prosoma darker; sternum orange-brown; opisthosoma grey to pale grey, darker on the back with 5-6 light transverse lines (Fig. 8a). Posterior eye line slightly recurved, eyes same size, equally spaced (about one diameter). Chelicerae with three promarginal teeth, the middle one the strongest, the third one more distant basally; three retromarginal minute teeth arranged in a group.
Legs: chaetotaxy 2222; tibiae I, position of first spine 0.29; one spine on Mt I in position 0.24–0.30 (0.27); position of trichobothrium on Mt I 0.131; trichobothrium on Mt IV absent. Sternum as long as wide.
Pedipalp (Fig. 8b-g): Patellar spine long and robust, tibia with a lateral spine. Paracymbium wide; proximal part with about ten hairs; posterior pocket inconspicuously pointed and only slightly sclerotized (Fig. 8e, PPO); anterior pocket with a broad, regularly rounded extension (Fig. 8e, APO); apical part digitiform and curved. Lamella characteristica sclerotized, median part narrow, curved 90° subdistally (visible in ventro-posterior view, Fig. 8c, LC), apical part flat, short in profile, distinctly widened and provided with fine indentations (Fig. 8e-g), two lobes visible in retrolateral view, the dorsal one slightly curved. Terminal apophysis small, flat, rather membranous at base, distal part slightly chitinous, widened and denticulated (Fig. 8b, TA). Suprategular apophysis very stout and strongly sclerotized, bifid crescent-shaped (Fig. 8b, SA), the ventral branch with a double indentation (visible in retrolateral view).
Female. Measurements (n = 6) (min.–max. (average)): total length 2.70–3.90 (3.38); PL 1.25–1.65 (1.46), PW 1.03–1.30 (1.17), PL/PW 1.22–1.27 (1.25); sternum length and width 0.68–0.90 (0.80); chelicerae 0.50–0.70 (0.63) long; Fe I 2.17–2.80 (2.50) long, Ti I 2.23–2.87 (2.57) long, Mt I 2.33 long.
Colour (based on specimens in alcohol): as in male, but opisthosoma grey to yellowish-grey with 5–6 clear transverse bands in the rear half more or less obvious and contrasted (Fig. 8h). Eyes: as in male, but PME barely closer together than PLE. Chelicerae with three promarginal teeth, the third one somewhat basally distant and slightly smaller; five retromarginal minute teeth, the last one of the series (the furthest from the fang joint) only barely larger. Legs: as in male but position of trichobothrium on Mt I 0.121–0.130 (0.125); Ti I position of second spine 0.73. Sternum: as in male.
Epigyne (Fig. 8j-l): Relatively simple, triangular in shape, as wide as long. In ventral view, the strongly domed proscape hides almost all the elements of the structure and particularly the median part of scape; posteriorly, only the distal part of the stretcher is visible. Depending on the longitudinal inclination and therefore the view angle, median part of scape may also be partially visible in ventral view (Fig. 8k).
Distribution and habitat. Lepthyphantes pieltaini is another species endemic to Morocco. As the type material was lost, one male (Kehf del Khashab Alqayqab cave) and two females (Toghobeit cave) among the new records will be deposited in the SMF. The species seems to occur very localized in the Rif Mountains and more precisely in an area between Chefchaouen and Bab Taza, including the Talassemtane National Park. Indeed, since its original description by Machado (1940), it has only been found once, in the same sector (Ribera 1983). Extensive research in several caves in the Moroccan Atlas (e.g. Barrientos et al. (2020) with more than 200 caves explored) did not yield any records of this species there. The present work adds nine new localities, located between 1260 m up to 1780 m a.s.l., to the range of the species. Barrientos et al. (2020) referred to Lepthyphantes pieltaini as an exclusively troglobiont species. However, it does not show any adaptations to subterranean life and as Bosmans (1985) indicated, it could perhaps be found outside caves. However, as yet, this is not the case, probably due to a lack of investigations, especially of habitats likely to be suitable for these sciaphilous species (e.g. wood litter in northern mountainous areas, gullies or north-facing scree). The specimens collected in this field study were all found in the area near the entrance (caves or shafts).
Figure 29 shows the distribution map of Lepthyphantes pieltaini, including the caves “Caf Muley Abdelkader” and “El Ajmas” (Machado 1940). According to www.geonames.org, the first is located in the province of Chefchaouen near the Chrafate spring ( www.chefchaouen.ma) (35.0666°N, -5.1000°W). Another location, Aframano Jerba cave (Tazza) (Ribera 1983), can be located approximately because of the mention of the locality in the tribal area El Ajmas (35.11666°N, -5.25000°W).
Comments. Following Bosmans (1985), L. pieltaini shows some affinities with the afer-complex of Lepthyphantes s. lat. (Saaristo & Tanasevitch, 1993). Bosmans pointed out the presence of a short lamella characteristica in the male and the absence of lateral lobes on the median part of the scape in the female. We consider that L. pieltaini globally fits the features that characterize the afer-complex according to Brignoli (1971), which we translate as follows according to the terminology of Saaristo & Tanasevitch (1996): for the female, circular or cordiform proscape, conspicuous distal part of scape and a stretcher which considerably protrudes; for the male, lamella characteristica relatively large, rather short, not forked nor pointed. Hormiga & Ribera (1990) stated that the terminal apophysis and the lamella characteristica of L. pieltaini are similar to those of L. bidentatus Hormiga & Ribera, 1990 (a species known only from Spain, WSC 2023); we do not support this assessment (see Diagnosis). The authors based their analysis on the drawings of Machado (1940), which are established on a palp that seems to be partially expanded and does not show all the details of the paracymbium.
Fig. 13.
Centromerus prudens, female. a. dorsal view; b. epigyne; c. vulva, dorsal view (photos: P. Oger)

Fig. 14.
Lepthyphantes aelleni. a. male, dorsal view; b. male pedipalp, retrolateral view; c. idem, ventro-retrolateral view; d. idem, ventro-prolateral view; e. idem, prolateral view; f. female, dorsal view; g. epigyne, ventral view; h. idem, lateral view (photos: P. Oger)

Fig. 15.
Lepthyphantes taza, female. a. dorsal view; b. epigyne, ventral view; c. idem, lateral view (photos: P. Oger)

Fig. 16.
Palliduphantes banderolatus, female. a. dorsal view; b. lateral view; c. epigyne, ventral view; d. idem, lateral view; e. idem, aboral view (photos: P. Oger)

Textrix Sundevall, 1833 (Agelenidae)
Textrix maroccana Lecigne spec. nov. (Fig. 9a-k)
Type material. Holotype: 1 ♂, MOROCCO: Azilal, Tisli N'khlil (La mine) cave (TIS) (31.95811°N, -6.10311°W, 1297 m a.s.l.), hand collecting, 16. Sep. 2017, leg. S. Moutaouakil. Remark: right legs I, II, IV missing; left leg I partially detached, as well as left pedipalp. Type material will be deposited in the SMF.
Etymology. The name of the species refers to “Morocco”, in reference to the endemism of this new cave-dwelling species.
Diagnosis. Textrix maroccana spec. nov. is classified in Textrix based on the shape of the prosoma, the ocular arrangement, the height of the clypeus, the dentition of both the anterior and posterior margins of the chelicerae as well as the conformation of the spinnerets (de Blauwe 1980, Almquist 2005). Currently, only six species are recognized as members of the genus (Textrix caudata L. Koch, 1872, Textrix chyzeri de Blauwe, 1980 only known in Hungary and parts of the Balkans, Textrix denticulata (Olivier, 1789), Textrix nigromarginata Strand, 1906 only known from Ethiopia, Textrix pinicola Simon, 1875 and Textrix rubrofoliata Pesarini, 1990), with none from Morocco (WSC 2023). Given the shape of the RTA and the conductor, Textrix maroccana spec. nov. does not resemble, and is not related to, any of these six species.The female remains unknown.
Description. Male. Measurements (n = 1): total length 5.3; PL 3.0, PW (maximal) 2.0, PL/PW 1.5; cymbium 1.37 long. Colour (based on specimens in alcohol): prosoma, legs, sternum and spinnerets pale yellowish, cephalic part of the prosoma barely darker; chelicerae brown; opisthosoma greyish brown. Cheliceral promargin with 3 teeth, the middle one the largest; cheliceral retromargin with 2 teeth widely spaced.
Male palp (Fig. 9b-j): cymbium pale yellowish, slender and long (Fig. 9i); RTA straight, slightly wider at the base (better visible in postero-retrolateral view), then evenly and finely tapering, pointing in posterior direction (Fig. 9d, i); embolus filiform, ovoid, originating at a 7 o'clock position, tip at a 5 o'clock position, apically curved to fit behind the terminal part of the conductor (Fig. 9g-h); conductor: its anterior part small scale-shaped, median part with a thin prolateral translucent extension (Fig. 9e, arrow), terminal part horn-shaped pointing prolaterally (Fig. 9d, g, arrow, h).
Female. Unknown.
Distribution. Only known from one cave (Tisli N'khlil) in the High Atlas (Azilal), the type locality.
Noteworthy records and species new for the spider fauna of Morocco
Agelenidae
Lycosoides parva (Denis, 1954) (Fig. 10a-c)
Previous citations. Bosmans et al. (2022).
Comments. Lycosoides parva is endemic to Morocco and only known from the Tazekka National Park, where both males and females were discovered. The male was described for the first time in Bosmans et al. (2022). We provide here figures of the pedipalp (Fig. 10a-c) for completion. The records mentioned by later authors indicate that occurences of L. parva are more bound to forest environments (Quercus-Cedrus) than caves, unlike most of the other species mentioned here. Its presence in the Ghar Bouslama may be a coincidence (a trogloxene species?).
Amaurobiidae
Amaurobius barbarus Simon, 1911 (Fig. 11a-e)
Identification. Lehtinen (1967): figs 203, 206; Bosmans (2021): p. 874, figs 4-7.
First record. MOROCCO: Taza (province), Grotte Blanche (ghar Bied/Ain EL Aouda), 1 ♂, hand collecting, 30. Oct. 2018, leg. B. Lebreton & A. Ouaziz.
Comments. So far, Amaurobius barbarus was only known from Algerian coastal habitats located mainly in forested environments and from Jerez de la Frontera in southern Spain (Bosmans 2021). Our Moroccan specimen was found in a cave in the Tazekka National Park, far from the coast (a trogloxene species?) and is the first record of the species in this country.
Hersiliidae
Tama edwardsi (Lucas, 1846) (Fig. 12a-c)
Identification. Rheims & Brescovit (2004): p. 212, fig. 45.
Material examined. MOROCCO: Oriental (region), Jlida 2 cave, 1 ♀, hand collecting, 2. Oct. 2019, leg. S. Moutaouakil.
Comments. Tama edwardsi is a ground-dwelling spider. Currently, it is the only species assigned to this genus. It was described by Lucas (1846, as Hersilia edwardsii) from Algeria in the vicinity of Oran, where it was observed under large stones in an area of ravines. It was recorded for the first time in Spain in 1936 and subsequently in Portugal in 1940 (Ribera et al. 1988), however, no details on its ecology were provided by these authors. It was recently mentioned for Morocco (Mousaid & Bouihouline 2023) from calcareous rock formations. Our discovery represents the second record of the species for this country.The specimen was recorded from the entrance of a cave (a trogloxene species?) near the Algerian border.
According to Le Péru (2011), the species is found in “Plant communities with Quercus ilex and Juniperus oxycedrus.” The ecology and distribution of the species remains poorly known and new records should always be accompanied by detailed habitat data.
Fig. 18.
Holocnemus reini. a. male, dorsal view; b. idem, lateral view; c. idem, ventral view; d. pedipalp, retrolateral view; e. idem, anterior view; f. idem, prolateral view; g. female, lateral view; h. idem, ventral view; i. idem, epigyne (Photos a-f, h-i: P. Oger; g: S. Moutaouakil)

Fig. 19.
Maghreba aurouxi, male. a. ventral view; b. dorsal view; c. frontal view; d. pedipalp, retrolateral view; e. idem, anterior view; f. idem, prolateral view (Photos a: S. Moutaouakil; b-f: P. Oger)

Linyphiidae
Centromerus prudens (O. Pickard-Cambridge, 1873) (Fig. 13a-c)
Identification. Bosmans (2006b): p. 131, figs 21-24; Nentwig et al. (2023); Oger (2023).
Previous citations (Morocco). Bosmans (1986, 2006).
Material examined. MOROCCO: Taza (province), Bouslama cave, 1 ♂, 1 ♀, 4 jj, hand collecting, 30. Oct. 2018, leg. B. Lips. Chefchaouen (province), Ghar Gharnaji cave (Kehf de Ouad N'Ghir), 2 ♀♀, 1 j, hand collecting, 29. Sep. 2019, leg. S. Moutaouakil.
Comments. Centromerus prudens is a widely distributed species, known from Norway to North Africa and from Iceland to central Russia (WSC 2023). It colonises forest and shrubby environments. In the Maghreb, the species is currently known from Algeria and Morocco where it is found at altitudes of 580 m up to 1850 m a.s.l. (Bosmans 1986, 2006b). Our new observations confirm its presence in the mountainous massifs of northern Morocco (1260 to 1510 m a.s.l.), where we found it in caves in the Talassemtane Park and the Tazekka National Park (trogloxene species). In France, it was observed up to 2300 m a.s.l. (AsFrA, pers. comm.)
Lepthyphantes aelleni Denis, 1957 (Figs 14a-h, 30)
Identification. Tanasevitch (2014): p. 282, figs 9-13.
Previous citations. Denis & Dresco (1957), Bosmans (2006a), Barrientos et al. (2020).
Material examined. MOROCCO: Taza (province), Bouslama cave, 2 ♀♀, hand collecting, 30. Oct. 2018, leg. B. Lips. Idem, Ghar Admam cave, 2 ♀♀, hand collecting, 01. Nov. 2018, leg. J. Lips. Idem, Ghar L'Ma cave (Grotte de l'eau), 1 ♂, 8 ♀♀, hand collecting, 19. Apr. 2019, leg. S. Moutaouakil. Idem, 5 ♀♀, hand collecting, 19. Apr. 2019, leg. J.-P. Degletagne. Idem, 1 ♂, 2 ♀♀, hand collecting, 23. Sep. 2019, leg. S. Moutaouakil.
Comments. Lepthyphantes aelleni is a Moroccan endemic species that was, until recently, only known from two caves located in the Tazekka National Park (Taza province). Subsequently, surveys of two other caves (Kef el Maa and Ifri Bouslama) in the national park also revealed the presence of the species. We recovered it in these two caves and added a third (Ghar Admam). Only the locality at Takerboust (Kef Pigeons; Barrientos et al. 2020), located nearly 200 km to the north-east of the other caves, is clearly different due to its remoteness and its position outside the Middle Atlas.
Figure 30 shows the distribution map of Lepthyphantes aelleni, established on the basis of available data (approximations of the historical records): “Gouffre de kaf el Bouk” (Denis & Dresco 1957) considering that this cave is located in the commune of Bab Bou Idir (34.066°N, -4.116°W), Taza province; “Gouffre du Friouato” (34.100°N, -4.066°W) (Bosmans 2006a, Barrientos et al. 2020) near Daya Chiker (Tanasevitch 2014); Kef el Maa, Taza (Barrientos et al. 2020); Kef Pigeons, Takerboust (34.800°N, -2.400°W) (Barrientos et al. 2020); Ifri Bouslama, Bab Bou Idir (34.083°N, -4.100°W) (Barrientos et al. 2020).
Lepthyphantes taza Tanasevitch, 2014 (Fig. 15a-c)
Identification. Tanasevitch (2014): p. 286, figs 22-24.
Previous citations. Tanasevitch (2014), Barrientos et al. (2020).
Material examined. MOROCCO: Taza (province), Chaâra cave, 1 ♀, hand collecting, 5. Jul. 2018, leg. S. Moutaouakil & Y. Elkassimi.
Comments. Lepthyphantes taza is endemic to the Moroccan Middle Atlas Mountains and currently only known from Tazekka National Park. It has recently been described from three females from a cave southwest of Taza (“ifri Tselet, cave near Ain Teslit, Châra”; Tanasevitch 2014). The species was also found at several places in the same cave system of Chaâra (“Cv. Trou de la Piste, Tabhairte”; “Ifri Azokhage (sic, [Ifri Azoggagh or Ifri Azoggarh]) (= Riviere Chara)”, same locality), which allowed the description of the male (Barrientos et al. 2020). The Ifri Azoggagh cave (Barrientos et al. 2020) and the Chaâra cave belong to the same cave system that runs along the Chaâra riverbed. It was not possible to exactly locate the former.
Figs 20-23.
20. Pritha sp., dorsal view. 21a-h. Lessertia barbara. a. male, dorsal view; b. male, pedipalp, ventro-retrolateral view; c. idem, prolateral view; d. tibial apophyses, dorsal view; e. female, dorsal view; f. idem, epigyne, ventral view; g. idem, epigyne, posterior view; h. idem, vulva, dorsal view. 22. Microctenonyx subitaneus, epigyne. 23. Mesiotelus mauritanicus, epigyne (photos 20-22: P. Oger; 23: S. Lecigne)

Fig. 24.
Maghreba kahfa, male. a. male, dorsal view; b. idem, frontal view; c. male, pedipalp, prolateral view; d. idem, anterior view; e. idem, retrolateral view. f. female, ventro-lateral view; g. idem, dorsal view; h. idem, ventral view; i. idem, frontal view; j. epigyne; k. vulva, dorsal view (photos 24a-e, g-k: P. Oger; 24f: J. Lips)

Figs 25-27.
25. Tegenaria pagana, male. a. pedipalp, ventral view; b. idem, retrolateral view; c. idem, retrolateral tibial apophysis. 26. Lepthyphantes fadriquei, female. a. dorsal view; b. epigyne; c. idem, lateral view. 27. Lepthyphantes maurusius, female. a. dorsal view; b. epigyne, ventral view; c. idem, lateral view (photos: P. Oger)

Palliduphantes banderolatus Barrientos, 2020 (Figs 16a-e, 31)
Identification. Barrientos et al. (2020): p. 19, fig. 11D-F.
Previous citations. Barrientos et al. (2020).
Material examined. MOROCCO: Agadir (province), Grotte des Chauves-souris, 2 ♀♀, hand collecting, 28. Oct. 2019, leg. J. Lips (coll. P. Oger). Idem, 1 ♀, hand collecting, 30. Oct. 2019, leg. Y. Znagui. Idem, 3 ♀♀ (2 ♀♀ coll. P. Oger), hand collecting, 30. Oct. 2019, leg. J. Lips. Youssoufia (province), Echemmaia, Karkar, 1 ♂, 1 ♀, hand collecting, 5. May. 2021, leg. S. Moutaouakil.
Comments. Palliduphantes banderolatus has been described only recently. It is known to date from two areas: north of Guelmim (type locality: Ifri N'Yzme cave, Taroudant province, Antiatlas) and in several caves in the karstic complex of the Puits Cochrisco (C.A. 9 to 11, Aghroud, Agadir). Our recent observations (Grotte des Chauves-souris, Karkar) add two localities for the species north of the two previously known ones; the outline of the range of P. banderolatus remains to be clarified. Figure 31 shows the distribution map of P. banderolatus, established on the base of available data (approximation of the historical records) (Barrientos et al. 2020).
Scotargus pilosus Simon, 1913 (Fig. 17a-d)
Identification. Bosmans (2006b): p. 150, figs 77-79.
First record. MOROCCO: Chefchaouen (province), Haffa Gazdir well, 1 ♀, hand collecting, 22. Aug. 2018, leg. S. Moutaouakil.
Comments. In the Maghreb, Scotargus pilosus has only been recorded in Algeria, where it was observed in high-altitude forests (1450 up to 1850 m a.s.l.). It is otherwise widely distributed in Eurasia and North Africa; some records were made in caves at high altitudes (Bosmans 2006b). The species is new to Morocco.
Pholcidae
In addition to the species mentioned in the paragraph below, several specimens of Pholcidae (see Appendix, Tab. S1) turned out to be species new to science.They will be described later.
Holocnemus reini (C. Koch, 1873) (Fig. 18a-i)
Identification: Huber (2022): p. 29-33, figs 62-76.
Previous citations (Morocco). Huber (2022).
Material examined. MOROCCO: Taza (province), Bouslama cave, 2 ♂♂, hand collecting, 30. Oct. 2018, leg. B. Lips. Idem, 1 ♀, 31. Oct. 2018, leg. B. Lebreton. Idem, 1 ♂, leg. S. Moutaouakil. Idem, 6 ♀♀, 20. Apr. 2019, leg. S. Moutaouakil. Idem, Ghar Admam cave, 3 ♂♂, 1 ♀, 01. Nov. 2018, leg. J. Lips. Idem, 1 ♂, 2 ♀♀, 23. Sep. 2019, leg. S. Moutaouakil. Oujda (region), JL1, 1 ♀, hand collecting, 2. Oct. 2019, leg. S. Moutaouakil.
Comments. Holocnemus reini is very closely related to H. caudatus. We relied mainly on the distance between the inner edge of the epigynal pockets (about 0.5 mm, Fig. 17i). This distance is about 0.24 to 0.3 mm for H. caudatus (after Huber 2022: p. 40, figs 102, 104 and 106). The difference in separating the males of the two species (the distances between male cheliceral apophyses) was more difficult to assess. Holocnemus reini is cited from Morocco, Algeria and Tunisia. Records of H. caudatus from Morocco (Nentwig et al. 2023) need to be verified.
Fig. 28-32.
Collecting localities of several endemic species from Morocco; open circle = loc. typ.; open triangles = citations; solid triangles = new records. 28. Lepthyphantes leknizii. 29. Lepthyphantes pieltaini. 30. Lepthyphantes aelleni. 31. Palliduphantes banderolatus. 32. Maghreba aurouxi (source: Shorthouse 2010)

Magreba aurouxi (Barrientos, 2019) (Figs 19a-f, 32)
Identification: Barrientos et al. (2019): p. 5, figs 2-5.
Previous citations. Huber (2022).
Material examined. MOROCCO: Errachidia (province), Aziza cave, 2 ♂♂, 5 jj, hand collecting, 11. Dec. 2020, leg. S. Moutaouakil. Idem, 1 ♂, 1 ♀, 08. Oct. 2022, leg. S. Moutaouakil.
Comments. The species was recently transferred from the genus Holocnemus (Huber 2022). Maghreba aurouxi is currently known from Morocco and is probably a Moroccan endemic species. Excluding the historical record between Irherm and Tiferki (“assigned tentatively” according to Huber 2022), the range of the species is so far restricted to the northeast of the Moroccan High Atlas (Fig. 32).
Discussion
The surveys that led to the results presented here did not have the specific aim of carrying out an araneological survey of the visited caves.They were mainly scientific internships intended for the geological study of mountains and, in particular, the karstology, the cartography and topography of cave environments. However, these internships were also intended to study the organisms living in these environments.
Over the whole period about forty caves were visited. Twenty-four spider species were recorded: fifteen are considered endemic to Morocco, of which about half are troglobionts. Furthermore, seven species proved to be new to science and the description of four of them is presented here. Three further species will be the subject of a later descriptions or require the collection of more material; four other samples revealed the presence of further anophthalmic specimens and suggest as many additional endemic species for Morocco and probably as many new species for science as presented here.
On a more global scale, the work still to be carried out in Moroccan caves is immense. For example, the Tazekka National Park (TNP), covering an area of almost 14000 ha, is rich in remarkable habitats such as very old forest formations formed mainly by Cedrus atlantica. There are 365 caves in and around the National Park, of which only 220 have been surveyed (out of the 1000 caves discovered to date in Morocco) (M. Amrani Marrakchi, pers. comm.). Exploration of several of them has already revealed the existence of further endemic spider species, e.g. Lepthyphantes leknizii (Barrientos 2020), Lepthyphantes taza (Tanasevitch 2014) and Lycosoides parva (Bosmans et al. 2022).
Based on the analysis of the spider fauna alone, our results highlight the outstanding biodiversity in particular of the Atlas Mountains, as already shown by Barrientos et al. (2020). This confirms the high importance of cave-dwelling ecosystems for biodiversity conservation on a global scale due to their high level of endemism. Subterranean habitats are under increasing pressure caused by ongoing climate change (Vaccarelli et al. 2023), are underrepresented in protected areas (Colado et al. 2023) and are in urgent need of further research (Nanni et al. 2023).
As previously explained (Lecigne & Moutaouakil 2021), protection of the Imi Ougoug cave system is necessary to conserve the ecosystems of these caves and its species, including Agraecina agadirensis Lecigne, Lips, Moutaouakil & Oger, 2020, an eyeless species, and Dysdera agadirensis spec. nov. The present work also highlights the urgent need to propose an extension of legal protection to other caves. The Ghar Admam cave seems particularly relevant in this context considering the presence of Lepthyphantes leknizii there (see above), an eyeless species strictly endemic to Morocco and so far known from this cave only, where it is most likely endemic. Such single-site endemics are often found in cave environments and are especially vulnerable to anthropogenic disturbances and climate change (e.g., Nitzu et al. 2018, Ni-miller et al. 2013). It is worth noting that other troglobiont specimens were also recorded at Ghar Admam, but could not be identified as they were immature (e.g., potential Leptonetidae and Liocranidae). Considering a systemic assessment, it would be worthwhile to examine, among the known and sufficiently surveyed caves (see above the caves of the TNP), those that would require such protection with regard to both the richness of their subterranean fauna and a presence of troglobiont/endemic species, which would make them highly vulnerable to anthropogenic disturbances.
We hope that our research stimulates further research in Moroccan cave systems and leads to the legal protection of more subterranean habitats in Morocco, together with their unique and often endemic fauna.
Acknowledgements
Our special thanks go to Pierre Oger for providing once again the photos to illustrate the text of most of the species recorded as well as help in identifying or confirming several species including Lepthyphantes aelleni, L. pieltaini, L. taza and Palliduphantes banderolatus, Robert Bosmans for confirming in particular Centromerus prudens and Scotargus pilosus, and José Antonio Barrientos for the transmission of information on Lepthyphantes leknizii.
We would also warmly thank all the collectors, without whom this work could not be carried out; they greatly contribute to the improvement of the knowledge of the spider fauna of Morocco. Special thanks are due to Youness el Kassimi, Youssef Znagui, Ouanaim Abderrahman and Abdelhamid Bahbaz for their assistance during fieldwork. We are grateful to the French Speleology Federation, who sponsored the two internships (2018 and 2019), and particularly its organizer Marc Latapie, as well as the Spanish association Biosp and the Barcelona History Museum (MUHBA).
We are also indebted to Robert Bosmans, the anonymous reviewers, the editorial team of the journal and especially Tobias Bauer for its recommendations and suggestions and more globally for improving the paper.
References
Appendices
Appendix
Tab. S1:
List of all records resulting from the present study. They are presented in alphabetical order of family and genus. The name of the locality, the location, the cave (code, see Tab. 1) and the date are specified, as well as species which are new for Morocco (#) and which are (re)described in the present paper (x)

Continued