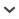The southern hemisphere water scavenger beetle genus Cylorygmus Orchymont, 1933 (Coleoptera: Hydrophilidae: Cylominae) is revised. Three species are recognized, one in Chile and two in South Africa. The morphological differences indicate that the African species are not congeneric with the Chilean one. Relictorygmus gen. nov. is established for the African R. trevornoahi sp. nov. (type species) and R. repentinus (Hebauer, 2002), both known from few localities in the Western Cape province of the Republic of South Africa. The genus Cylorygmus with the only species C. lineatopunctatus Orchymont, 1933 is endemic to a small region in central Chile. Its larva is described in detail based on specimens collected in association with adults. Both genera and all species are diagnosed, described and illustrated, and an identification key for adults is provided. Our study demonstrates that the trans-Atlantic disjunct distribution of Cylorygmus was based on inaccurate taxonomic treatment and did not reflect the real evolutionary history of these beetles.
Introduction
Organisms with widely disjunct distribution are frequent model groups for biogeographers since they help us to reconstruct the history of landmasses and their past interconnections, and to understand the processes which led to modern distributional patterns (e.g., Brundin 1966, de Quieroz 2014). The beetle family Hydrophilidae, a diverse group containing about 3000 known species, includes some examples of putatively disjunct generic distributions: Austrotypus Fikáček et al., 2014 occurring in Peru and Australia (Fikáček et al. 2014) and Cetioeyon Hansen, 1990 found in Papua New Guinea and Suriname (Fikáček and Short 2010) are the most recently published examples. Five more genera with African-American disjunct distribution are known in the primarily terrestrial subfamilies Cylominae and Sphaeridiinae. Four of them, the cylomine genus Cylorygmus Orchymont, 1933, coelostomatine genera Phaenonotum Sharp, 1882 and Cyclotypus Sharp, 1882, and the omicrine genus Omicrus Sharp, 1879 are mainly distributed in the Neotropics, but few African species were also assigned to them by Bameul (1992), Régimbart (1907) and Hebauer (2002): Cylorygmus repentinus Hebauer, 2002 from South Africa, Phaenonotum africanum Régimbart, 1907 from Cameroon, Omicrus hebaueri Bameul, 1992 from the Ivory Coast, and two species of Cyclotypus from Madagascar. The African species of these genera were not reviewed taxonomically, and their generic placement requires confirmation (Seidel and Fikáček personal observation). The North American megasternine species Tectosternum naviculare (Zimmerman, 1869) is the only case which has been reviewed in detail. Smetana (1978, 1984) confirmed its assignment to the genus Tectosternum Balfour-Browne, 1958, previously considered being an African endemic.
The aforementioned genus Cylorygmus is the only African genus belonging to the subfamily Cylominae, a small assemblage of ‘relict’ genera distributed in the southern hemisphere (Seidel et al. 2016) and having the highest genus and species diversity in New Zealand and Australia, with several endemic genera in austral South America (Fikáček et al. 2014, Fikáček and Vondráček 2014, Minoshima et al. 2015). Cylorygmus was originally described from Chile with the single species C. lineatopunctatus Orchymont, 1933. Hebauer (2002) added C. repentinus, based on two specimens collected in the Western Cape Province of South Africa. This discovery was not only the first record of the subfamily Cylominae in Africa, but it also implied a trans-Atlantic disjunct distribution for the genus Cylorygmus. The assignment of the African species to Cylorygmus was only based on overall morphological similarity, lacking a thorough comparison to C. lineatopunctatus, and was hence considered as requiring confirmation (e.g., Fikáček et al. 2014). To facilitate the revision of its generic placement, we organized an expedition to Western Cape in 2015. Surprisingly, the specimens we collected revealed to be related to C. repentinus, but representing another, as yet undescribed, species. These fresh specimens also allowed us to test the generic placement of the African species of Cylorygmus, of which the morphological and taxonomical implications are discussed in this paper. We are also summarizing all know data about the Chilean Cylorygmus, including the description of its larva, based on specimens collected in association with adults.
Material and methods
Adult morphological examination. For generic character examination, specimens were dissected after short treatment in 10% KOH (in case of C. lineato-punctatus) or after extracting the dissolved internal tissues in the solution of 180 µl of Qiagen ATL and 20 µl of proteinase K, bleached in 15% hydrogen peroxide solution and mounted on permanent slides in Euparal. For habitus illustration, partially focused photographs were taken using Canon EOS 550D digital camera with attached Canon MP-E65 mm R2.8 1–5× macro lens, followed by a combination in Helicon Focus software. Photographs of slide-mounted parts were taken using Canon EOS 1100D digital camera attached to an Olympus BX41 compound microscope and subsequently combined in Helicon Focus software. SEM micrographs were taken using a Hitachi S-3700N environmental electron microscope at the Department of Paleontology, National Museum (Prague, Czech Republic). All pictures were subsequently adapted in Adobe Photoshop CS6 (e.g., cleaning background, cropping). Original unedited photos, including those only used for comparative purposes and not included into this paper were submitted as a .zip file to Zenodo archive under the doi: 10.5281/zenodo.1065382.
Larval morphological examination. The methodology for larval examination followed Minoshima and Hayashi (2011). The specimens were cleared using 10% KOH solution, dissected and mounted on H-S Slides (Shirayama et al. 1993) with Euparal resin or preserved in ethanol. Observations and dissections were carried out using an Olympus SZX12 stereoscopic microscope and Nikon Eclipse E600 compound light microscope. Illustrations were made with the aid of a drawing tube attached to the E600. Line drawings were prepared using the software Paint tool SAI (Systemax Inc., Japan) and Photoshop CC (Adobe Systems Inc., USA). Habitus photographs were taken using the same methodology and equipment as adult ones. The morphological terminology of larva generally follows Archangelsky (1997) and Minoshima and Hayashi (2011), for terminology of antennal segments we followed Beutel (1999). For the chaetotaxy of the larval head we referred to Fikáček et al. (2008) and Bytte-bier and Torres (2009). Following abbreviations are used:
AN
antenna,
FR
frontale,
gAN
group of antennal sensilla,
gAPP
group of sensilla on inner appendage of maxilla,
gFR
group of sensilla on frontale,
gLA
group of sensilla on labium,
gMX
group of sensilla on maxilla,
L1, L2, L3
first, second and third instar,
LA
labium,
MN
mandible,
MX
maxilla,
PA
parietale,
SE
sensorium.
Specimen depositories. Examined material is housed in the following collections:
BMNH
Natural History Museum, London, United Kingdom (M. Barclay);
CDTB
collection of D. T. Bilton, Plymouth, United Kingdom;
FMNH
Field Museum of Natural History, Chicago, Illinois, USA (A. Newton, C. Maier);
HNHM
Hungarian Natural History Museum, Budapest, Hungary (O. Merkl);
ISAM
Iziko South African Museum, Cape Town, South Africa;
JBCC
Juan E. Barriga collection, Curico, Chile;
KMNH
Kitakyushu Museum of Natural History and Human History, Kitakyushu, Japan (Y. Minoshima);
MNNC
Museo Nacional de Historia Natural, Santiago de Chile, Chile (M. Elgueta);
NHMW
Naturhistorisches Museum Wien, Wien, Austria (M. Jäch);
NMPC
Department of Entomology, National Museum, Prague, Czech Republic (J. Hájek, M. Fikáček);
SEMC
Snow Entomological Museum, University of Kansas, Lawrence, USA (A. Short);
ZMHB
Museum für Naturkunde, Berlin (J. Frisch, B. Jäger).
Taxonomy
Key to species of Cylorygmus and Relictorygmus gen. nov.
1. Body dorso-ventrally compressed (Figs 1A–B). Labrum dorsally with a median group of stout setae (Fig. 3A). Gula narrowing anteriad to a half of its width at base (Fig. 3B); femora pubescent with intermixed stout setae (Figs 3D–E, G–I); pubescence of ventrite 1 intermixed with stout setae (Fig. 2K); abdominal apex with stout setae (Fig. 2L); male genitalia with median lobe ca. twice as wide as parameres, gonopore apical (Fig. 9A). Chile. Cylorygmus lineatopunctatus Orchymont
—. Body moderately convex (Figs 10B, E). Labrum without median group of setae dorsally (Fig. 12D). Gula narrowing anteriad to a fourth of its width at base (Figs 12F, K); femora pubescent, lacking intermixed stout setae (Figs 12A–C, I); pubescence of ventrite 1 without intermixed stout setae (Fig. 11K); abdominal apex without stout setae (Fig. 11L); male genitalia with median lobe at midlength ca. half as wide as parameres, gonopore subapical (Figs 9C, E); South Africa 2 (Relictorygmus gen. nov.)
2. Ventral surface of profemora densely pubescent at base (Fig. 12I); elevated anterior part of mesoventrite sub-triangular with weakly sinuate posterior margin (Fig. 10M), transverse ridge of mesoventrite straight (Figs 12L–M); anterior metaventral process narrowly triangular (Fig. 12L); male genitalia with parameres curved inwards in apical half, median lobe of aedeagus rather narrow at base, slightly narrowing apicad (Fig. 9C) Relictorygmus repentinus (Hebauer)
— Ventral surface of profemora sparsely pubescent at base (Fig. 12A); elevated anterior part of mesoventrite heart-shaped (Fig. 12H), transverse ridge of mesoventrite angulate (Fig. 12H); anterior metaventral process widely triangular (Fig. 12G); male genitalia with parameres relatively straight, median lobe of aedeagus basally ca. 2.0 × as wide as apical part narrowing anteriad to half of its width (Fig. 9E) Relictorygmus trevornoahi sp. nov.
Cylorygmus Orchymont, 1933
Type species. Cylorygmus lineatopunctatus Orchymont, 1933 (by original designation).
Differential diagnosis from co-occurring genera. In southern South America, Cylorygmus can be confused with Enochrus Thomson, 1859 both as adult and larva. Adult Cylorygmus differs from Enochrus in maxillary palps (Fig. 1C) bent inwards (in contrast to zigzag shaped palpomeres in Enochrus) and flattened mesoventrite (Fig. 3D) (in contrast to posteromedially raised mesoventrite forming a high keel or large cone-shaped tooth in Enochrus). Larvae can be distinguished from Enochrus by (1) the absence of prolegs on abdominal segments III–VII (Fig. 1E) (with spinose prolegs on these segments in Enochrus), and (2) the nasale with five very large and distinct teeth, epistomal lobes large and symmetrical (Figs 4C, 6A, 7C) (nasale with five to more irregular and much smaller teeth, epistomal lobes narrow, asymmetrical, right one projecting further than left one in Enochrus (see Archangelsky 2002 and Byttebier and Torres 2009). Larva of Cylorygmus also resembles the larva of Argentinian endemic genus Hydramara Knisch, 1925 which inhabits streams, but differs from it by mandibles with two retinacular teeth (with two large teeth and one small basal tooth in Hydramara) and by antennal sensorium well developed (minute and difficult to see in Hydramara).
Differential diagnosis from cylomine genera. Adult. Labrum (Figs 1C, 3A) exposed (in contrast to Adolopus Sharp, 1884, Andotypus Spangler, 1979, Anticura Spangler, 1979, Austrotypus, Coelostomopsis Hansen, 1990, Cyloma Sharp, 1872, Eurygmus Hansen, 1990, Petasopsis Hansen, 1990 and Rygmodus White, 1846 which have completely or largely concealed labrum); mesoventrite (Fig. 3D) not distinctly elevated posteromesally (in contrast to Adolopus, Andotypus, Borborophorus Hansen, 1990, Coelostomopsis, Cyloma, Exydrus Broun, 1886, Hydrostygnus Sharp, 1884 and Tormissus Broun, 1893 which have a posteromesal elevation), but with transverse ridge (in contrast to Anticura, Cylomissus Broun, 1903, Eurygmus, Pseudohydrobius Blackburn, 1898, Rygmodus, Rygmostralia Orchymont, 1933 and Saphydrus Sharp, 1884 which also have non-elevated mesoventrite, but lack the transverse ridge); first metatarsomere (Fig. 2I) shorter than second (in contrast to Andotypus, Austrotypus and Coelostomopsis with very long first metatarsomere); elytra not explanate (Figs 1A–B) and epipleura very narrow at apex (in contrast to Borborophorus and Petasopsis with explanate elytra and epipleura very wide throughout).
Cylorygmus differs from Relictorygmus gen. nov. in the following combination of characters: labrum dorsally with median group of stout setae (Fig. 3A); gula (Fig. 3B) moderately wide anteriorly (ca. half of its width at base); anterior margin of mentum (Fig. 3B) very weakly indented below the palpal insertions; femora (Figs 3G–I) pubescent with intermixed stout setae; mesoventrite (Fig. 3D) with weakly developed transverse groove posteromesally, setose centrally; anterior metaventral process narrow, about 2.3× longer than wide at base (Fig. 3D); abdominal ventrite I (Fig. 2K) with intermixed stout setae; posterior margin of abdominal ventrite V (Fig. 2L) with series of stout setae mesally; abdominal tergites VI–VII (Fig. 2J) subdivided mesally; male genitalia (Fig. 7A) with median lobe ca. twice as wide as parameres, gonopore apical.
Larva. The symmetrical wide mandibles, each with two retinacular teeth (Figs 5C, 8C), nasale with five large teeth and symmetrical epistomal lobes (Figs 4C, 6A, 7C), antennal sensorium shorter than antennomere 3 (Figs 5A–B, 8A–B), inner face of maxillary stipes with 5 setae only (Figs 5E, 8E), prementum only slightly narrower than mentum and with distinctly developed ligula (Figs 5G–H, 8G–H), abdomen without projections or tracheal gills (Figs 1D–E) and legs well developed (Figs 1G, 6B) diagnoses the larva of Cylorygmus easily from members of Amphiopini (mandibles slender, mentum very wide, ligula absent), Berosini (abdomen usually with projections or gills, mandibles asymmetrical or slender and symmetrical, epistomal lobes strongly asymmetrical or nearly absent), Laccobiini (mandibles and epistomal lobes either asymmetrical, or nasale with less than 5 large teeth), Hydrobiusini (mandibles with a small tooth below the basal retinacular tooth), Hydrophilini (head appendages long and slender, mentum much wider than prementum, abdomen with at least short projections), Chaetarthriinae (antennal sensorium as long as antennomere 3, ligula projecting further than labial palps, coronal sulcus usually absent) and Sphaeridiinae (nasale with less than 5 teeth, inner face of stipes mostly with many stout setae, legs often shortened to completely reduced). The larva may be hence confused only with those of the subfamilies Acidocerinae, Enochrinae and Cylominae, to some of which it is also very similar by external habitus. Larvae of all known Enochrinae differ from Cylorygmus by nasale with multiple irregularly shaped small toothlets (rather than five large well-defined teeth in Cylorygmus) and by the dorsal surface of mentum completely covered by cuticular spines (with two submedian bare areas in Cylorygmus). All known larvae of Acidocerinae differ from Cylorygmus by nasale with at least 6 teeth.
Within Cylominae, Cylorygmus can be easily distinguished from Anticura, Cylomissus, Andotypus and Austrotypus and Tormissus by nasale with 5 teeth and inner face of stipes with 5 setae only (with 1–2 teeth on nasale and multiple setae on inner face of stipes in the latter genera), and from Cyloma by nasale with 5 teeth (nasale with 3 teeth in Cyloma). All mentioned genera except Borborophorus differ in the configuration of the teeth of the nasale: Saphydrus with the median and lateral teeth large and the intermediate small, Rygmodus in both right and left tooth more widely isolated from the median three teeth (plus mandibles, maxillae and prementum very elongate), and Adolopus with all teeth grouped together (i.e. without any tooth more widely isolated or distinctly smaller than others). The supposed larva of Borborophorus is very similar to that of Cylorygmus in the form of nasale and epistomal lobes, as well as in most other characters examined (but slides were not done and chaetotaxy not examined), and seems to differ only by the shape of head capsule (slightly widened posteriorly, compared to parallel-sided in Cylorygmus), smaller stemmata and antennal sensorium as long as antennomere 3 (slightly shorter in first instar larva and much shorter in third instar larva in Cylorygmus).
Redescription of adult. Body elongate oval (Fig. 1A), compressed in lateral view (Fig. 1B). General coloration brown to black.
Head. Clypeus and frons (Fig. 1C) distinctly punctate; frontoclypeal suture very weakly marked, by lack of punctures; clypeus not excised above antennal insertions, anterior margin concave, marginal bead absent, surface with only few isolated trichobothria. Frons with trichobothria next to inner margin of each eye. Eyes (Figs 1B–C) small, slightly protruding from head outline; facets ca. 16–17 µm in diameter. Labrum (Figs 1C, 2B, 3A) well sclerotized, exposed in front of clypeus, visible in dorsal view, ca. 3.0× wider than long, bisinnate anteriorly, rounded laterally; anterolateral margin with long setae, anteromedian part with group of stout setae; epistome with two groups of long spine-like setae anteriorly and with two parallel series of long hair-like projections posteriorly. Mandibles (Fig. 2C) slightly asymmetrical, without developed mandibular angle, apex bidentate; prostheca with series of long projections, slender and elongate in anterior part, stout and shorter at base; outer margin sparsely pubescent; mola large, with small cuticular projections. Maxilla (Fig. 2A) with large sub-pentagonal cardo, lacking trichobothria; basistipes widely triangular, moderately pubescent, bearing one or two trichobothria; mediostipes vaguely defined from lacinia; lacinia membranous, with distally situated finger-like projection, pubescent, setae more thickened and rounded towards anterior part of lacinia and on the projection, galea long, apical membranous portion blunt at apex, densely pubescent, the pubescence regularly ordered in rows, setae thickened, bent and apically rounded; maxillary palpus four-segmented, palpomere 1 minute, palpomere 2 slightly swollen distally, about as long as palpomere 3 and 4 each; palpomere 4 without digitiform sensilla. Labium with submentum (Fig. 2E) about as long as mentum, pubescent; mentum (Fig. 3B) transverse, ca. 1.5 × wider than long, widening anteriad, anterior margin almost continuously rounded, very weakly indented below palpal insertions, surface flat, with a weak transverse groove parallel to anterior margin; lateral margins with dense series of setae; prementum (Fig. 2F) subdivided into two large lateral lobes bearing long, apically acute and slender setae, as well as short, apically rounded and thickend setae; palpiter weakly sclerotized; labial palpus with 3 palpomeres (Fig. 2F), basal palpomere minute, second palpomere widened, bearing numerous setae, palpomere 3 thin, only with few setae. Antenna with 9 antennomeres (Fig. 2D); scapus long and thin, widest at midlength; pedicel widest at midlength; antennomere 3 about 0.7 × as long as pedicel and as long as antennomeres 4–6 combined; antennomere 6 (cupule) small, slightly narrower than antennal club; antennomeres 7–9 forming a loosely segmented pubescent antennal club, antennomere 9 elongate ca. 1.7× as long as each of antennomeres 7–8. Gula wide (Fig. 3B), narrowing anteriad to a half of its width at base, with median ridge, tentorial pits reduced. Temporae (Fig. 3B) rugose and sparsely pubescent, with weakly defined ridge arising from inner margin of each eye.
Prothorax. Pronotum (Fig. 1A) widest at base (ca. 0.45× as long as wide, ca. 1.5× wider at base than in front angles), continuously arcuately narrowing anteriad; anterior angles acute (Figs 1B, 2G); dorsal punctation similar to that on frons; trichobothria present in anterolateral corner; anterior, posterior and lateral margins with narrow marginal bead. Hypomeron (Fig. 2G) with very narrow lateral glabrous part and densely pubescent mesal part divided by a very fine ridge; antennal grooves absent; hypomeral process simply pointed, not reaching prosternal process. Prosternum (Figs 2G, 3C) slightly shorter than procoxal cavity anteriorly to procoxae, flat, with weak transverse impression, angulate and beaded on anterior margin, with evenly distributed short setae; exposed part of prosternal process pointing between procoxae, posterior part concealed by procoxae slightly widened posteriorly, truncate at apex. Coxal cavities (Fig. 2G) closed internally, open posteriorly, coxal fissure long, widely open. Profurca short and weakly sclerotized, profurcal arms widely separated basally, in form of asymmetrical apically slightly widened stalks.
Mesothorax. Scutum with finely microsculptured median portion, bearing sparsely arranged setae laterally; scutellar shield (Fig. 1A) exposed, triangular, pointed posteriorly, as long as wide, bearing punctation similar to frons and a series of stout setae below the anterior margin. Elytron (Figs 1A–B) elongate, ca. 2.3× as long as wide, evenly convex; with 10 series of punctures, serial punctures distinctly larger than punctures in intervals; trichobothria present on alternate intervals (Fig. 3F); sutural stria present, adjacent to punctural series 1; scutellar stria absent; lateral margin of elytra beaded, very narrowly explanate, lateral margin bare, without stout setae; epipleuron wide at base, gradually narrowing towards level of metacoxae, then narrow but distinct, reaching elytral apex; lateral narrow bare part not delimited from mesal pubescent one by a ridge; ventral face of elytron without elevated ridges. Mesoventrite (Fig. 3D) divided from mesanepisterna by anapleural sutures; subtriangular in shape in anterior two thirds, narrowing anteriorly, widely extending in posterior third, lateral extensions with distinct coxal lobes; anapleural sutures arcuate; median part of mesoventrite flat, with rugose surface, without elevated projection, with weakly developed transverse groove posteromesally; anterior portion without anteromedian pit-like groove; mesoventral process narrow, abutting to metaventral process, not elevated above it. Mesanepisterna (Fig. 3D) not meeting mesally, very narrowly divided by anterior portion of mesoventrite; anterior collar well-defined, rather wide, sparsely pubescent along anterior bead. Mesepimeron with large ventral portion, not reaching anterior collar of mesanepisternum; forming lateral margin of mesocoxal cavity; whole surface with densely arranged setae. Mesocoxal cavities obliquely transverse, very narrowly separated from each other by meso- and metaventral processes; internal postcoxal wall developed mesally and posteriorly, narrow. Mesofurca well developed, rather short, furcal arm not developed; basal portion fused and rather wide, bifurcating only very dors ally into two arms, each with narrow asymmetrical plate-like apical extension.
Metathorax. Metanotum weakly sclerotized, ca. 2× wider than long, alacristae slightly diverging posteriad. Metaventrite (Fig. 3E) ca. 2.9× wider than long, slightly longer than mesoventrite, evenly convex, with median area bare and slightly raised posteriorly, margin of elevated area with thick long setae, discrimen reaching mid of metaventrite, surrounded by sparsely pubescent area, lateral part of metaventrite with dense short pubescence, interspersed with longer thick setae; anteromesal area with shallow impression; katepisternal suture weakly developed, vanishing towards lateral margin of mesoventrite, katepisternum narrow and bare; posterior metaventral process bifid. Metanepisternum ca. 4.5× longer than wide, with oblique ridge subanteriorly, anterior margin straight; surface pubescent. Metafurca (Fig. 2H) Y-shaped; stalk long, grooved, without basal extensions; arms long and wide, with apical plate-like extensions. Hind wing (Fig. 2M) well developed, widest at mid-length, ca. 1.6× longer than elytron, venation well-developed in basal portion, absent in distal part; anal lobe moderately large, defined by well-developed anal notch; RA tightly attached to ScA except subbasally, both reaching triangular radial cell, RA3+4 as long as radial cell, r4 arising from its midlength; distal portion of RP and MP1+2 forming a strong loop, distally with long median spur; vein complex of MP3+MP4+Cu+AA well developed, not connected to MP1+2 by cross-veins, with well-developed and completely closed basal and wedge cells; distal portion of MP4+CuA complex H-shaped, arising from anterior portion of wedge cell; AA4 long, reaching posterior margin; AP3+4 long, reaching over the midlength of anal lobe.
Legs. Mesotrochantin ca. 0.6× as long as mesocoxal cavity, narrow, exposed ventrally anterior of mesocoxae. Coxae: procoxae subglobular, pubescent ventrally; mesocoxae transverse, narrowly separated, pubescent ventrally; metacoxae transverse, finely pubescent except anterior part, all coxae with interspersed thick setae. Trochanters with proximal parts concealed by coxae, distal portion subtriangular, pubescent ventrally; meso- and metatrochanters bisinuate on posterior margin, posterior margin not continuous with that of femora. Femora attached to trochanters by their posertobasal (in meso- and metafemora) or anterobasal (in profemora) parts only; anterobasal (in meso and metafemora) or posterobasal (in profemora) part free, angulate; pubescence of ventral surface in profemora (Fig. 3G) dense and in meso and metafemora (Figs 3H–I) sparse; all femora with tibial grooves well defined by sharp ventral edges. Tibiae slightly longer than femora, slightly widening distally; each tibia with longitudinal series of setae on dorsal, ventral and lateral faces; apex with group of short to very long spines, protibia with two long, meso- and metatibia with one moderately long and one very long spurs mesally, the longer one ca. 1.5–2.0× as long as the first tarsomere. Tarsi (Fig. 2I) with 5 tarsomeres; protarsus with tarsomeres 1–4 short, subequal in length, tarsomere 5 longer, ca. as long as tarsomeres 1–3 combined; meso- and metatarsi with basal tarsomere shorter than tarsomere 2, tarsomeres 3 and 4 ca. subequal in length to tarsomere 1, tarsomere 5 ca. 2× longer than tarsomere 3; ventral surface of all tarsomeres with sparse long pubescence; claws small, simply arcuate.
Abdomen with five exposed ventrites; ventrites evenly convex, without median carina, densely pubescent; ventrite I (Fig. 2K) with intermixed stout setae; posterior margin of ventrite V (Fig. 2L) entire, with stout setae. Laterotergite III without stridulatory file; tergites VI–VII (Fig. 2J) subdivided mesally.
Genitalia. Aedeagus (Fig. 9A) of simple trilobed type; phallobase subequal in length to parameres, with wide, indistinctly defined, posteriorly rounded manubrium; parameres distinctly longer than median lobe; median lobe wider than parameres, weakly narrowing apicad, apex cut off and pubescent, gonopore distinct, apical. Sternite 9 (Fig. 9B) with wide median part constricted subbasally, widened and notched posteriorly, lateral struts shorter than median part. Female genitalia of the general morphology similar to other Hydrophilidae (see e.g. Fikáček 2010), with very longcoxostyli and gonostyli, otherwise not examined in detail.
Figure 1.
Cylorygmus lineatopunctatus Orchymont, 1933. (A–C) adult habitus: (A) dorsal; (B) lateral; (C) frontal view of the head. (D–H) habitus of third instar larva: (D) dorsal; (E) ventral; (F) detail of thorax and head in dorsal view; (G) detail of thorax and head in ventral view; (H) tergite on abdominal segment VIII.

Figure 2.
Detailed adult morphology of Cylorygmus lineatopunctatus Orchymont, 1933. (A) maxilla; (B) labrum; (C) mandibles; (D) antennae; (E) mentum and prementum; (F) detail of the labial palp; (G) prothorax in ventral view; (H) metafurca; (I) metatarsus; (J) abdominal tergites VI–VII; (K) detail of abdominal ventrite I with intermixed stout setae; (L) apex of ventrite V with stout setae; (M) hindwing.

Figure 3.
Cylorygmus lineatopunctatus Orchymont, 1933, details of adult morphology, SEM micrographs. (A) labrum, dorsal view; (B) head, ventral view; (C) prosternum; (D) mesothorax, ventral view; (E) metaventrite; (F) elytral interval with trichobothrium; (G–I) femora in ventral view: (G) profemur; (H) mesofemur; (I) metafemur.

Cylorygmus lineatopunctatus Orchymont, 1933
(Figs 1–8, 9A–B, 13)
Philhydrus lineato-punctatus Germain, 1911: 58 (nomen nudum).
Cylorygmus lineatopunctatus Orchymont, 1933: 293.
Type material examined. Neotype (designated by Moroni 1985): 1 male (MNNC): “Quillota [printed] / XI-96 [in red handwriting] // 810 // Colección / P. Germain [printed] // ♂ // Museo Nacional / Santiago-Chile // NEOTIPO [blue label, handwritten] // CHILE M.N.H.N. / Tipo No 3779 // Cylorygmus / lineatopunctatus D'Orch. 33 / Det. J. Moroni 1970″.
Comments on type material. There was long-lasting confusion about the identity of Cylorygmus lineatopunctatus. Orchymont (1933) described the genus and species according to a single female collected by P. Germain in Quillota in November 1896 deposited in the Hamburg Museum, which was however destroyed during the Second World War. Spangler (1974) associated the specimens collected in Magallanes (southern Chile) with Orchymont's (1933) description, designated a neotype and later described the larva of this taxon (Spangler 1979). He noted the large distance between the original type locality and that of his neotype, and cast doubt whether the original type locality is in the Valparaíso region near Santiago de Chile, or in Aysén Region in the south. Moroni (1985) examined the material collected by P. Germain deposited in MNNC including specimens which were likely collected together with the lost holotype, and realized that the specimens redescribed by Spangler (1974) in fact belonged to a different species [later it was found that Spangler's (1974) specimens in fact represent the genus Pseudorygmodus Hansen, 1999 and are not related to Cylorygmus; Hansen 1999, Fikáček and Vondráček 2014], Moroni (1985) recognized the invalidity of the neotype designation by Spangler (1974) since Spangler was not in compliance with Art. 75.3.6 (ICZN) which requires “evidence that the neotype came as nearly as practicable from the original type locality [Art. 76.1]”. Moroni selected the above specimen collected by P. Germain likely together with the original holotype as a neotype. We examined the specimen of C. lineato-punctatus from Orchymont collection in IRSNB mentioned by Hansen (1999): this specimen corresponds by the label data with the lost holotype as well as Moroni's neotype and bears Orchymont's identification label. It is moreover conspecific with Moroni's neotype and with additional specimens examined by us. Very likely all examined specimens including the lost holotype and the neotype by Moroni belonged to the same series of specimens from the Germain collection. This confirms that the identity of C. lineatopunctatus fixed by Moroni (1985) corresponds to the original meaning of the name by Orchymont (1933).
Additional adult material examined. CHILE: Región Metropolitana de Santiago: 4 spec. (MNNC): Hospital Lo Águila [33°54'S 70°46'W], without date and collector; 1♂, 4 spec. (MNNC): Lo Águila, Chile central // Lo Águila, 33°54′S 70°46′W, J. Moroni det., P. Germain coll.; 1♀, 7 spec. (MNNC): Santiago, Lo Águila, lgt. P. Germain; 1♂, 11 spec. (MNNC): Chile, P. Germain coll, [considered as collected in Lo Águila by Moroni]; 1 spec. (MNNC): Santiago, Rungua [33°0′24.12″S 70°53′20.4″W], 7.xi.[18]69 [remaining text illegible]; 2 spec. (MNNC): Maipo, Rangue [33°51′19″S 70°53′11″W], 4.vi.2004, lgt. M. Elgueta & M. Guerrero; 3 spec. (JBCC): Mina La Disputada [33°10′14″S 70°18′16″W], x.1989, J. E. Barriga lgt.; 1♀, 5 spec. (NHMW, NMPC): Quebrada La Plata [33°29′38″S 70°53′23″W] / Prov. Santiago de Chile /lg. H. Franz / Sa 6–7; 1 spec. (FMNH): Peñalolén 14 km SE of Santiago [33°33′21″S 70°31′41.3″W], 7.x.1945, lgt. L. Peña Guznián; 1♂, 20 spec. (HNHM, NMPC): Farellones, 6.x.1965, Hungarian Soil-Zool. Exp. Nr. P-B.36, Andrássy, Balogh & Mahunka lgt. [further collecting details provided by Andrássy et al. (1967): Farellones, 2100 m, singled in surroundings, but always along streamlets, mostly beneath stones], Valparaíso Región: 1♀ (IRSNB): Chile, Quillota / XI. 1896 // A. d'Orchymont det. / Gylorygmus / lineatopunctatus // Para- / type // Coll. R. I. Sc. N. B.; 1 spec. (MNNC): Valparaiso / Quillota [32°52'S 71°15′0″W] // Philydrus / lineatopunctatus P. G. [= Ph. Germain ] / Paulo / Quillota // lineatopunctatus P. G. ined. 1911; 2 spec. (MNNC): Quillota [32°52′S 71°15′0″W] / XI-96 // Chile / Valparaíso / Quillota 11.1896 // castaneno P. G. ined. 1627; 1♂, 1 spec. (MNNC): Quillota, ii.[1897], lgt. P. Germain; 1♀ (MNNC): Quillota, xi.[18]96, lgt. P. Germain [labelled as topotype by Moroni]; 1♀ (MNNC): 810 // Quillota xi.96 // TOPOTIPO; 15 spec. (MNNC): Quillota [32°52′S 71°15′0″W], without date and collector; 8 spec. (FMNH): La Vega vicinity, 33°2.71′S 71°1.63′W (ANMT1014), 940 m a.s.l., sclerophyll forest with Eucalyptus, berlesate damp litter in dry stream bed, 15.xii.1996, lgt. A. Newton & M. Thayer (FMHD #96-207); 3 spec. (FMNH): Parque Nacional La Campana (Sector Granizo), Cajón La Opositora, 32°58.78′S 71°6.93′W (ANMT1045), 685 m a.s.l., sclerophyll forest with Nothofagus obliqua, berlesate wet debris at stream, 29.xii.2002, lgt. M. Thayer & A. Newton (FMHD#2002-101); 1 spec. (FMNH): Parque Nacional La Campana (Sector Ocoa), vicinity of Quebrada Buitrera, 32°55.89′S 71°5.1′W (ANMT 1047), 415 m a.s.l., sclerophyll woodland with Jubaea chilensis palms and Trichocereus cacti, in cow dung near stream edge, 28.xii.2002, lgt. M. Chani; 3 spec. (FMNH): same locality, but in debris at pool in dry creek bed, 28.xii.2002, lgt. D. Clarke (FMHD#2002-100); 3 spec. (FMNH): same locality, but in moss at base of tree at stream edge, 28.xii.2002, lgt. M. Chani; 2 spec. (FMNH): same locality, but wet debris at stream edge, 28.xii.2002, lgt. A. Solodovnikov; 10 spec. (FMNH, NMPC, KMNH): same locality, but berlesate from flood debris at small stream, 30.xi.2002, lgt. M. Thayer, A. Newton, A. Solodovnikov & D. Clarke (FMHD#2002-026); 1♂ (FMNH): 4 km E of Quebrada Alvarado, 33°3′S 71°3′W (ANMT668.2), 500 m a.s.l., gallery forest along stream, wet leaves, stream edge, 5.i.1983, lgt. A. Newton & M. Thayer; 1♂, 2 spec. (NMPC): Parque Nacional La Campana (Sector Ocoa), 4.75 km SE of park entrance, “La Cascada”, 32°57.7′S 71°3.2′W, 870 m a.s.l., sifting of wet/humid flood debris and leaf litter at sides of the pool below the waterfall, 20.xi.2013, lgt. M. Fikáček, D. Vondráček & P. Kment (CH03); 1♂ (HNHM): Between Concon and Quintero, 14.xii.1965, Hungarian Soil-Zool. Exp. Nr. P-B.300, Mahunka lgt. [more details from Andrássy et al. 1967: “netted from trees and bushes”].
Larval material examined. CHILE: Valparaíso Reglón: 2 first instar larvae, 2 likely second instar larvae, 4 third instar larvae (FMNH, NMPC, KMNH: 1 L1 and 2 L3 dissected and examined in detail): Parque Nacional La Campana (Sector Ocoa), vicinity of Quebrada Buitrera, 32°55.89'S 71°5.1'W, berlesate from flood debris at small stream, 30.xi.2002, lgt. M. Thayer, A. Newton, A. Solodovnikov & D. Clarke (FMHD#2002-026). The larvae were collected together with adults of Cylorygmus and two early-instar larvae of Enochrus (Hugoscottia) sp., no other hydrophilids co-occurred in the sample. Adults of Enochrus (H.) fulvipes (Solier, 1849) were found by us in similar habitats on several localities in La Campana National Park in 2013, which also indicates that adults and larvae of Cylorygmus and Enochrus (Hugoscottia) co-occur in the same microhabitat in the region. Larvae of all other hydrophiloid genera occurring in Chile (see Jerez and Moroni 2006) except of that of Cylorygmus are known (e.g., Archangelsky 1997), and may be easily diagnosed from the examined larvae. Hence, despite not being able to confirm the identification of the larvae by means of DNA barcoding, we consider the identification of the larvae by association with adults and by exclusion of other Chilean genera as reliable.
Further published records. CHILE: Reglón Metropolitana de Santiago: Peñalolén [33°33′21″S 70°31′41.3″W], 7.x.1945, lgt. L. Peña (Moroni 1985); Maipú, 15.xi.1982, lgt. R. Honour (Moroni 1985)
Redescription of adult. 4.4–5.1 mm long, 2.2–2.5 mm wide (ca. 1.9× as long as wide), elongate oval, compressed in lateral view. Coloration (Figs 1A–C). Dorsal coloration dark brown to black; margins of pronotum yellow to light brown; ventral coloration dark brown, only hypomeron, epipleuron and legs light brown. Head. Eyes (Figs 1B–C) moderately small, interocular distance ca. 5.5× the transverse diameter of eye. Mentum (Fig. 3B) with anterior margin evenly rounded mesally, slightly concave laterally, surface becoming rugose and moderately setose towards lateral margins. Prothorax. Prosternum (Fig. 3C) mesally with moderately long setae, setae evenly distributed, surface of anterior marginal bead rugose, anterior portion with a weak transverse impression. Mesothorax. Mesoventrite (Fig. 3D) with weakly developed transverse groove posteromesally, not reaching coxal cavities, surface posterior of transverse groove sparsely setose; elevated anterior part of mesoventrite sub-triangular with straight posterior margin. Metathorax (Fig. 3E). Raised median area of metaventrite sparsely setose; anterior metaventral process narrow, elongated, ca. 2.3× as long as maximum width. Legs. Ventral surface of profemora (Fig. 3G) densely pubescent at base, bare at apex; mesofemora (Fig. 3H) sparsely pubescent with intermixed stout setae; metafemora (Fig. 3I) sparsely pubescent with only few intermixed stout setae. Male genitalia. Parameres (Fig. 9A) weakly arcuate on outer face, rounded at apices; median lobe ca. 2× as wide as parameros in ventral view, apex cut off and pubescent, slightly constricted subapically, gonopore apical.
Description of larvae. Third Instar. General morphology (Figs 1D–E). Body slender, widest between abdominal segments 2–3. Colour. Head capsule (Figs 1D–G) reddish brown with yellowish anterolateral part; appendage yellowish, only mandibles reddish brown. Sclerites (Figs 1D–H) on thorax and abdomen reddish brown, legs (Fig. 1G) yellowish brown; membranous parts slightly infuscate yellowish white, median part of abdominal segments 1–7 with one pair of oblong reddish brown patches dorsally (Fig. 1D).
Head. Head capsule subquadrate (Fig. 6A), widest medially. Cervical sclerites subquadrate. Frontal lines lilyform, coronal line very short. Surface of head capsule smooth. Six stemmata on each anterolateral portion of head capsule. Posterior tentorial pits present on median part close to junction of gular and submental sulci. Clypeolabrum (Fig. 7C) slightly asymmetrical. Nasale asymmetrical, with five distinct teeth; right four teeth aggregated, right upward; left one not projecting further than fight four teeth. Lateral lobes of epistome present, almost symmetrical but right lobe slightly projecting further than left lobe. Antenna (Figs. 8A–B) 3-segmented, slender; surface of antenna bare. Antennomere 1 widest, somewhat longer than antennomere 2, antennomere 3 the shortest and narrowest. Antennal sensorium present. Mandibles (Figs. 8C–D) moderately stout, symmetrical. Two inner teeth present on median part of inner face each; apical inner tooth slightly larger than proximal one. Maxilla (Figs. 8E–F) slender, 6-segmented, longer than antenna. Cardo moderate in size, irregularly shaped. Stipes the longest, ca. 1.7 times as long as palpomeres 1–4 combined; tooth-like cuticular projections present basally on inner face. Maxillary palpus short, 4-segmented. Palpomere 1 widest, incompletely cylindrically sclerotised, partly membranous dorsally. Inner process sclerotised. Palpomere 2 short, wider than palpomeres 3 and 4; palpomere 3 longest, wider than palpomere 4; palpomere 4 rather short and narrowest. Labium (Figs. 8G–H) well-developed. Submentum fused to head capsule, transverse; submental sulcus indistinct. Mentum trapezoidal, widest at base. Dorsal and lateral surface densely covered with small cuticular teeth, dorsal surface partly bare. Prementum subquadrate, without cuticular teeth. Ligula slender, mostly sclerotised, strongly curved towards dorsally, ca. as long as labial palpomere 2. Labial palpus slender, long, palpomere 1 wider and much shorter than palpomere 2; palpomere 2 long; intersegmental membrane between palpomere 1 and 2 bearing several cuticular projections dorsally.
Thorax (Figs 1F–G). Membranous parts very densely covered with short, hair-like, cuticular projections. Prothorax wider than head capsule. Proscutum formed by one large plate subdivided by fine sagittal line, anterior and posterior margins weakly sclerotised; proscutal plate covered with setae of variable length and densely distributed fine cuticular structures. Prosternal sclerite incompletely subdivided by fine sagittal line at posterior half; bearing numerous short setae on anterior part. Mesonotum with three pairs of dorsal sclerites; two pairs on anterior margin, median pair transverse, each attached mesally, lateral pair small; one large pair behind anterior pairs, subpentagonal, subdivided by transverse ridge and shallow transverse groove; each attached mesally. A pair of spiracles on anterolateral face, projecting laterally. Metanotum with three pair of dorsal sclerites; one pair on anterior margin, transverse, attached mesally, lateral pair very small, almost reduced. One large pair behind anterior pair, transverse, subquadrate, anterior halves shallowly concave; last pair behind large sclerite, transverse. Legs (e.g., Fig. 6B) rather short and slender, 5-segmented; all three pairs similar in shape.
Abdomen (Figs 1D–E, H). Abdomen 10 segmented, widest at segments 2 and 3, then slightly tapering posteriad; membranous parts covered with densely arranged short cuticular projections. Segment 1 with two pairs of small dorsal sclerites medially; posterior pair bearing seta. One pair of spiracles on lateral part of dorsal surface, tuberculate. Segments 2–7 similar to segment 1, but with one pair of small dorsal sclerites. Spiracular atrium (Fig. 6C): Segment 8 with large, oval dorsal plate (Figs 1H, 6C). Procercus short, partly sclerotised. Segment 9 trilobed. Lateral lobe of spiracular atrium large, widest basally, narrowing apically; inner part partly sclerotised; acrocercus absent, or stout and short present on ventral face of lateral lobe. Median lobe of spiracular atrium large, partly sclerotised, almost parallel-sided; a pair of slightly projecting lateral projection on basal part of median lobe.
First instar (Figs 4–5, 6B). Similar to third instar larvae, but sclerites weakly pigmented than third instar. Thorax. Prothorax as wide as or slightly wider than head capsule. Dorsal sclerites on meso- and metanotum weakly visible, lateral pair on mesonotum may be absent.
Chaetotaxy of head. First instar. Frontale (Figs 4A, C). Central part with three pairs of sensilia (FR1-3) slightly divergent posteriad; FR1 long seta; FR2 pore-like, situated more anteriorly and more mesally to FR1; FR3 very short seta, anteriorly to FR2. Pore-like sensilium FR4 and setae FR5–6 located posteromesally to antennal socket; FR5 stout, short seta, posteromesally to FR6, FR6 long seta, laterally to FR4–5; FR4 anteriorly to FR5. FR7 rather long seta, situated on inner face of antennal socket. Setae FR9–10 mesally to antennal socket; FR9 long, FR10 rather long. Pore-like sensilium FR15 and rather long, paddle shaped seta FR8 situated mesally on clypeolabrum, behind nasale; from FR15 anteriorly to FR8. Sensilla FR11–14 situated on epistome, anteromesally to antennal socket; FR11, 13–14 pore-like, FR12 short seta; FR11–13 closely aggregated, situated on inner part of epistome, FR12 posteriorly to FR11 and FR13, FR11 mesally to FR1213. FR14 located close to inner margin of antennal socket. Nasale with a group of six stout and short setae (gFR1); two minute ventral sensilla on laterally to median tooth of nasale. Epistomal lobe with stout short setae on anterior margin, composed by two groups; nine (six lateral, three mesal; lateral-most seta missing in the figured specimen) on left lobe, seven (five lateral, two mesal) on lateral lobe. Parietale (Figs 4A–B) Dorsal surface with a group of five sensilla (PA1–5) forming longitudinal row in posterior part; PA1–2 and 4–5 very short setae, PA3 pore-like. PA6 pore-like, located posteromesally close to coronal line. Sensilla PA7, 12–17 situated medially on dorsal to lateroventral surface, forming irregularly transverse row; from dorsal to ventral, PA7, PA12, 13, 14, 15, 16, 17. PA7 very long, paddle shaped; PA12 rather long seta; PA13 very long, on laterodorsal face; PA14 long seta, paddle shaped; PA15 and PA17 pore-like, PA16 long seta. Pore-like sensillum PA10 and short seta PA11 located between anterior and posterior rows of stemmata; PA10 mesally to PA11. PA8 very long seta, situated behind outer part of antennal socket. PA9 long seta, paddle shaped, on outer margin of antennal socket. PA19–22 located laterally on anterior corner of head capsule, closely aggregated; PA19 pore-like, PA20 rather short seta, PA21 and PA22 very long setae; from dorsal to ventral, PA19, 20, 21, 22. Pore-like sensilla PA23–25 on ventral mandibular articulation; PA23 on outer part; PA25 on inner part, PA24 between PA23 and PA25. Very long seta PA18 situated posteriorly to PA15–17, pore-like sensilla PA30 posteriorly to PA18. PA26–28 located median part of ventral surface of parietale; PA26 very long seta, anteriorly to PA27–28; PA27 pore-like, between PA26 and PA27; PA28 seta. PA29 pore-like, situated posteromesal part of ventral face of parietale. Antenna (Figs 5A–B). Antennomere 1 with five pore-like sensilla (AN1–5). AN1 situated laterally on median part of inner surface; AN2 dorsally on mesal part of anterior third; AN3–5 subapically, AN3 on lateral face, AN4 on inner face, AN5 ventrally on mesal part. Antennomere 2 with one pore-like sensillum (AN6) situated dorsally on subapical part of sclerite and five seta (AN7–11) dorsally on intersegmental membrane between antennomeres 2 and 3, and sensorium (SE1). Setae AN7–9 on outer face, posteriorly and close to SE1, AN7 and AN8 short, AN9 very short; AN7 anteriorly to AN8, AN9 mesally to AN7–8. AN10–11 on inner face, AN10 very long, paddle shaped; AN11 short. Sensorium SE1 on outer face, stout, slightly shorter than antennomere 3. Antennomere 3 with group of apical sensilla (gAN) in apical membranous area. Mandibles (Figs 5C–D). Three pore-like sensilla (MN2–4) on median part; MN4 situated on dorsolateral face, anterolaterally to MN2–3, anteriorly to MN1; MN2 posterolaterally to MN3. Rather long seta MN1 placed on lateral face, behind MN4; MN5 on apical third of lateral face. MN6 undetectable. Positions of sensilla identical in both mandibles. Maxilla (Figs 5E–F). Cardo with one ventral seta (MX1). Inner face of stipes with a row of five stout setae (MX7–11); simple seta MX7 at base, MX8–11 with small apical tooth, at equal intervals. Pore-like sensilla MX2–3 situated ventrally, MX2 laterally on posterior third, MX3 mesally on midlength. Pore-like sensilla MX4 and setae MX5–6 lateroventrally on subapical part of sclerite; MX5 long, MX6 very long. Dorsal surface of palpomere 1 with one rather short, stout seta (MX16) situated basally on inner face. Three sensilla (MX12–14) located laterally on distal part of ventral surface of sclerite; MX12 pore-like, MX13–14 very long seta; MX12 dorsally to MX13–14, MX13 between MX14 and MX12. Pore-like sensilla MX15 and MX17 situated on membrane behind inner appendage, MX17 dorsally, MX15 ventrally. Inner appendage with one long, paddle shaped seta and a few short setae apically (gAPP). Palpomere 2 with two pore-like sensilla (MX18–19); MX18 ventrally on lateral part; MX19 dorsomesally on intersegmental membrane between palpomeres 2 and 3; MX27 undetectable. Palpomere 3 with two pore-like sensilla (MX20 and MX22), and very long setae (MX21 and MX23). MX22 located ventrally on median part of sclerite; MX20–21 and MX23 distally on borderline between sclerite and intersegmental membrane; MX21 ventrally on inner face, MX23 dorsally on outer face, MX20 laterodorsally. Palpomere 4 with one rather long seta (MX24) situated subbasally on inner face, and with digitiform (MX25) and pore-like (MX26) sensilla apically on outer face of sclerite; MX25 dorsally to MX26. Apical membranous area of palpomere 4 with several minute setae (gMX). Labium (Figs 5E–H). Submentum with two pairs of setae (LA1–2); LA1 very long on lateral corner, LA2 short on anterolateral corner. Ventral surface of mentum with one pair of very long setae (LA3) and porelike sensilla (LA4) on anterolateral part; LA3 behind LA4, LA4 subapically. Prementum and its anterior membranous area with five pairs of sensilla (LA5–9). LA5–7 situated lateroventral surface of prementum, minute seta LA5 at base, extremely long seta LA6 on midlength, pore-like sensillum LA7 apically on sclerite, close to borderline between sclerite and membrane. Pore-like sensilla LA8–9 situate median part of dorsal surface; LA8 on subbasally, LA9 situated on anterior membranous area. Ligula with one pair of very long setae (LA10) and two pairs of pore-like sensilla (LA11– 12); LA10 dorsally at base; LA12 dorsally at apex, LA11 ventrally on subbasal part. Palpomere 1 with two sensilla (LA13–14); LA13 minute seta, situated basally on lateral part of ventral surface of sclerite; LA14 porelike, dorsally on median part of intersegmental membrane between palpomeres 1 and 2. Palpomere 2 with one pore-like sensillum LA15 situated apically on outer face; several setae of variable shape (gLA) on apical membranous area.
Third Instar. Frontale (Figs 7A, C). One small secondary seta situated posteriorly to FR5, laterally to FR2–3; one very short secondary seta behind antennal socket. Parietale (Figs 7A–B). Slightly oblique longitudinal row of short secondary setae present along frontal line. Two rather short secondary setae close to PA5. Longitudinal row of short, irregularly arranged secondary setae situated between PA7 and PA17; two minute secondary setae anterior to PA18; one short secondary seta anterior to PA17. Two secondary sensilla between PA9 and PA19, mesal one seta, lateral one pore-like. Few minute secondary setae on anterior corner of head capsule. Antenna (Figs 8A–B) without secondary sensilla. Mandibles with minute secondary setae on posterior half of lateral face; a few basal secondary sensilla longer than others. Maxilla (Figs 8E–F) with secondary sensilla on stipes. Lateral face with two moderately short secondary setae on anterior third and posterior third respectively. One long secondary seta close to MX 5–6, undetectable from the primary setae. Ventral face with four short secondary setae on inner part, almost equidistant; anterior seta close to MX11, posterior seta close to MX9, but positions of the sensilla variable. Labium with secondary setae on mentum: 8 to 9 stout secondary setae of variable length present laterally, three moderately long setae on anterior margin.
Distribution. The species has very restricted distribution. Actual records are known between latitudes of ca. 33°S and 34°S in the Metropolitan and Valparaiso Region in Chile central Chile (Fig. 13A). It occurs both in the coastal range and in the foothills of the Andes, up to ca. 2100 m of altitude. The reconstruction of the potential distribution based on climatic data was performed by Fikáček and Vondráček (2014) and indicated that the species may be potentially distributed from northern parts of Valparaíso Region to northern O'Higgins only, i.e. in the region which is strongly influenced by agriculture. Since it represents a very isolate and ancient clade of the Hydrophilidae (see Discussion), having a very restricted distribution, C. lineatopunctatus surely deserves protection.
Natural history. All known specimens were collected from September to February, i.e. during the summer season in Chile. Most adults and all larval specimens were collected from humid to wet debris, leaf litter and moss, in or along stream beds in sclerophyll or Nothofagus forests (Figs 13C–E). Incidentally, the specimens are probably hiding in other wet places near streams; for example, there were also found in cow dung near the edge of a stream.
Figure 4.
Head capsule of the first instar larva of Cylorygmus lineatopunctatus Orchymont, 1933. (A) head capsule in dorsal view; (B) head capsule in ventral view; (C) clypeolabrum.

Figure 5.
Head appendages of the first instar larva of Cylorygmus lineatopunctatus Orchymont, 1933. (A-B) antenna: (A) dorsal view; (B) ventral view; (C–D) mandibles in dorsal view: (C) left one; (D) right one; (E–F) maxilla: (E) dorsal view; (F) ventral view, cardo omitted; (G–H) mentum and prementum: (G) dorsal view; (H) ventral view.

Figure 6.
Larva of Cylorygmus lineatopunctatus Orchymont, 1933. (A) head in dorsal view; (B) mesothoracic leg in anterior view; (C) abdominal segments VIII–X in dorsal view. (A, C) - 3rd instar larva, (B) 1st instar larva.

Figure 7.
Head capsule of the third instar larva of Cylorygmus lineatopunctatus Orchymont, 1933. (A) head capsule in dorsal view; (B) head capsule in ventral view; (C) clypeolabrum.

Figure 8.
Head appendages of the third instar larva of Cylorygmus lineatopunctatus Orchymont, 1933. (A–B) antenna: (A) dorsal view; (B) ventral view; (C–D) mandibles in dorsal view: (C) left one; (D) right one; (E–F) maxilla: (E) dorsal view; (F) ventral view; (G–H) mentum and prementum: (G) dorsal view; (H) ventral view.

Figure 9.
Aedeagus (A, C, E) and male sternite 9 (B, D, F) of Cylorygmus and Relictorygmus species. (A-B) Cylorygmus lineatopunctatus Orchymont, 1933; (C–D) Relictorygmus repentinus (Hebauer, 2002), holotype; (E–F) Relictorygmus trevornoahi sp. nov., paratype.

Figure 10.
Habitus of Relictorygmus species. (A–C) R. repentinus (Hebauer, 2002), paratype: (A) dorsal habitus; (B) lateral habitus; (C) frontal view of the head. (D–F) R. trevornoahi sp. nov., paratype: (A) dorsal habitus; (B) lateral habitus; (C) frontal view of the head.

Relictorygmus gen. nov.
Type species. Relictorygmus trevornoahi gen. et sp. nov. (by present designation).
Etymology. The genus name is formed from the Latin adjective relictus (abandoned) and the core -rygmus which refers to Cylorygmus to which species of the new genus were assigned until now. The name refers to the genus being a remnant and supposed last member of the cylomine fauna in Africa. The gender is masculine.
Differential diagnosis from co-inhabiting genera. In South Africa, Cylorygmus may be confused with Enochrus and Coelostoma Brullé, 1835. Relictorygmus differs from Enochrus in maxillary palps (Figs 10C, F) bend inwards (in contrast to zigzag shaped palpomeres in Enochrus) and flattened mesoventrite (Figs 12H, M) (in contrast to posteromedially raised mesoventrite forming a high keel or large cone-shaped tooth in Enochrus). It differs from Coelostoma by the flat mesoventrite (Figs 12H, M) (in contrast to mesoventrite with high arrow-head shaped elevation and anterior pit-like groove in Coelostoma), and metatarsomere 1 shorter than metatarsomere 2 (Fig. 11I) (much longer than metatarsomere 2 in Coelostoma).
Differential diagnosis from cylomine genera. Relictorygmus gen. nov. corresponds to Cylorygmus in most external characters, but differs from it in the following combination of characters: labrum dorsally without median group of stouter setae (Fig. 12D); gula narrow anteriorly (Figs 12D, K) (ca. one fourth of its width at base); mentum with anterior margin markedly indented below palpal insertions (Figs 12F, K); femora pubescent but lacking intermixed stout setae (Figs 12A–C, I); mesoventrite (Figs 12H, M) with well-developed transverse groove posteromesally, pubescent centrally; anterior metaventral process wide and triangular (Figs 12G, L); abdominal ventrite I (Fig. 11K) without intermixed stout setae; posterior margin of abdominal ventrite V (Fig. 11L) without stout setae mesally; abdominal tergites VI–VII (Fig. 11J) not subdivided mesally; male genitalia (Figs 9C, E) with narrow median lobe, gonopore subapical.
Description of adult (characters marked by an asterisk were not examined for R. repentinus). Body (Fig 10) widely elongate, moderately convex. General coloration dark brown.
Head. Clypeus and frons (Figs 10C, F) distinctly punctate; frontoclypeal suture very weakly marked, by lack of punctures; clypeus not excised above antennal insertions, anterior margin concave, marginal bead absent, surface with only few isolated trichobothria. Frons with trichobothria next to inner margin of each eye. Eyes (Figs 10B–C, E–F) moderately large, slightly protruding from head outline; facets ca. 16–17 µm in diameter. Labrum (Figs 10C, F; 11C, 12D) well sclerotized, exposed in front of clypeus, visible in dorsal view, ca. 3.3× wider than long*, bis innate anteriorly, rounded laterally; anterolateral margin with long setae, dorsal surface with transverse series of slightly stouter setae; epistome with two groups of long spinelike setae anteriorly and with two parallel series of long hair-like projections posteriorly. Mandibles* (Fig. 11C) slightly asymmetrical, without developed mandibular angle, apex bidentate; prostheca with series of long projections, slender and elongate in anterior part, stout and shorter at base; outer margin sparsely pubescent; mola large, with small cuticular projections. Maxilla (Fig. 11 A) with large sub-pentagonal cardo, lacking trichobothria; basistipes widely triangular, moderately pubescent, bearing one or two trichobothria; mediostipes vaguely defined from lacinia; lacinia* membranous, with distally situated finger-like projection, pubescent, setae more thickened and rounded towards anterior part of lacinia and on the projection, galea* long, apical membranous portion blunt at apex, densely pubescent, pubescence regularly ordered in rows, setae thickened, bent and apically rounded; maxillary palpus four-segmented, palpomere 1 minute, palpomere 2 slightly swollen distally, about as long as palpomere 3 and 4 each; palpomere 4 without digitiform sensilla. Labium with submentum (Figs 12E, K) about as long as mentum, pubescent; mentum transverse, ca. 1.3–1.4× wider than long, slightly widening anteriad, anterior margin rounded mesally (Figs 12F, K), surface moderately concave, with weak transverse groove parallel to anterior margin; lateral margins with sparse series of setae; prementum (Fig. 11E–F) subdivided into two large lateral lobes bearing long, apically acute and slender setae, as well as short, apically rounded and thickend setae; palpiter weakly sclerotized; labial palpus with 3 palpomeres (Fig. 11F), basal palpomere minute, second palpomere widened, bearing numerous setae, palpomere 3 thin, only with few setae. Antenna with 9 antennomeres (Fig. 11A); scapus long and thin, widest at midlength; pedicel widest at mid-length; antennomere 3 about as long as pedicel and as long as antennomeres 4–6 combined; antennomere 6 (cupule) small, slightly narrower than antennal club; antennomeres 7–9 forming a loosely segmented pubescent antennal club, antennomere 9 elongate ca. twice as long as each of antennomeres 7–8. Gula (Figs 12F, H) wide, narrowing anteriad to a fourth its length at base, with median ridge, tentorial pits reduced. Temporae (Figs 12H, F) rugose and sparsely pubescent, with weakly defined ridge arising from inner margin of each eye.
Prothorax. Pronotum (Figs 10A, D) widest at base (ca. 0.45× as long as wide, ca. 1.5–1.6× wider at base than in front angles), continuously arcuately narrowing anteriad; anterior angles acute; dorsal punctation similar to that on frons; trichobothria present in anterolateral corner; anterior, posterior and lateral margins with narrow marginal bead. Hypomeron (Fig. 11G) with very narrow lateral glabrous part and densely pubescent mesal part divided by a very fine ridge; antennal grooves absent; hypomeral process simply pointed, not reaching prosternal process. Prosternum (Figs 11G, 12E, 12J) slightly shorter than procoxal cavity anteriorly to procoxae, flat, with or without weak transverse impression, arcuate and beaded on anterior margin, mesally with sparsely arranged moderately long setae; exposed part of prosternal process pointing between procoxae, posterior part concealed by procoxae slightly widened posteriorly, truncate at apex*. Coxal cavities* (Fig. 11G) closed internally, open posteriorly, coxal fissure long, widely open. Profurca* short and weakly sclerotized, profurcal arms widely separated basally, in form of asymmetrical apically slightly widened stalks.
Mesothorax. Scutum* with finely microsculptured median portion, bearing sparsely arranged setae laterally; scutellar shield (Figs 10A, D) exposed, triangular, pointed posteriorly, as long as wide, bearing punctation similar to frons and a series of stout setae below the anterior margin. Elytron elongate (Figs 10A–B, D–E), 2.3–2.4× as long as wide, evenly convex; with 10 series of punctures, serial punctures distinctly larger than punctures in intervals; trichobothria absent in all intervals; sutural stria present, adjacent to punctural series 1; scutellar stria absent; lateral margin of elytra beaded, very narrowly explanate, lateral margin bare, without stout setae; epipleuron wide at base, gradually narrowing towards level of metacoxae, then narrow but distinct, reaching elytral apex; lateral narrow bare part not delimited from mesal pubescent one by a ridge; ventral face without elevated ridges. Mesoventrite (Figs 12G–H, L–M) divided from mesanepisterna by anapleural sutures, slowly narrowing anteriorly (suture well defined), subtriangular in shape in anterior two thirds, widely extending in posterior third, lateral extensions with distinct coxal lobes; anapleural sutures arcuate, converging but not meeting anteriad; median part of mesoventrite flat, without projection, with moderately developed transverse groove posteromesally; anterior portion without anteromedian pit-like groove; mesoventral process narrow, abutting to metaventral process, not elevated above it. Mesan-episterna (Figs 12G–H, L–M) not meeting mesally, very narrowly divided by anterior portion of mesoventrite; anterior collar well-defined, rather wide, sparsely pubescent along anterior bead. Mesepimeron with large ventral portion, not reaching anterior collar of mesanepisternum; forming lateral margin of mesocoxal cavity; whole surface with densely arranged setae. Mesocoxal cavities obliquely transverse, very narrowly separated from each other by meso- and metaventral processes; internal postcoxal wall developed mesally and posteriorly, narrow. Mesofurca* well developed, rather short, furcal arm not developed; basal portion fused and rather wide, bifurcating only very dorsally into two arms, each with narrow asymmetrical plate-like apical extension.
Metathorax. Metanotum* weakly sclerotized, ca. 2× wider than long, alacristae slightly diverging posteriad. Metaventrite (Figs 12G, L) ca. 2.9× wider than long, slightly longer than mesoventrite, evenly convex, with raised median area, median area more distinct posteriorly, margin of elevated area bearing same setae as metaventral surface, discrimen reaching mid of metaventrite, surrounded by sparsely pubescent area, lateral part of metaventrite with dense short pubescence, without interspersed thick setae; anteromesal area with small to wider shallow impression; katepisternal suture weakly developed, vanishing towards lateral margin of mesoventrite, katepisternum narrow and asetose; posterior metaventral process bifid. Metanepisternum ca. 3.7× longer than wide, with oblique ridge subanteriorly, anterior margin straight; surface pubescent. Metafurca* (Fig. 11H) Y-shaped; stalk long, grooved, without basal extensions; arms long and wide, with apical plate-like extensions. Hind wing (Fig. 11M) well developed, widest sub-basally, ca. 1.6× longer than elytron*, venation well-developed in basal portion, absent in distal part; anal lobe large, defined by well-developed anal notch; RA tightly attached to ScA except subbasally, both reaching triangular radial cell, RA3+4 as long as radial cell, r4 arising from its midlength; distal portion of RP and MP1+2 forming a strong loop, distally with long median spur; vein complex of MP3+MP4+Cu+AA well developed, not connected to MP1+2 by cross-veins, with well-developed and completely closed basal and wedge cells; distal portion of MP4+CuA complex H-shaped, arising from anterior portion of wedge cell; AA4 long, reaching posterior margin; AP3+4 long, reaching over the midlength of anal lobe.
Legs. Coxal surface without interspersed thick setae; femora (Figs 12A–C, I) ventrally with sparse to dense pubescence lacking intermixed stout setae. In other characters corresponding to those of Cylorygmus.
Abdomen with five exposed ventrites; ventrites evenly convex, without median carina, densely pubescent; ventrite I (Fig. 11K) without intermixed stout setae; posterior margin of ventrite V (Fig. 11L) entirely without stout setae; laterotergite III without stridulatory file; tergites VI–VII (Fig. 11J) not subdivided mesally.
Genitalia. Aedeagus (Figs 9C, E) of simple trilobed type; phallobase subequal in length to parameres, with wide, weakly to distinctly defined posteriorly rounded manubrium; parameres slightly longer than median lobe; median lobe narrower than parameres, gonopore distinct, subapical. Male sternite 9 (Figs 7D, F) and female genitalia as in Cylorygmus.
Figure 11.
Detailed adult morphology of Relictorygmus trevornoahi sp. nov. (A) antenna (distal part of antennal club not in horizontal position); (B) maxilla (maxillary palpus missing) and detail of galea; (C) labrum; (D) mandibles; (E) mentum with prementum; (F) detail of labial palp; (G) prothorax in ventral view; (H) metafurca; (I) metatarsus; (J) abdominal tergites VI–VIII; (K) detail of abdominal ventrite I without setae; (L) apex of abdominal ventrite V without stout setae; (M) hindwing.

Relictorygmus repentinus (Hebauer, 2002) comb. nov.
Cylorygmus repentinus Hebauer, 2002: 11.
Type material examined. Holotype: male (ZMHB): “R. S. Africa 17.xi.1993/34°27′S/20°24′E, leg. Uhlig / Cape Province: / De Hoop Nat. Res. / lake shore, reed sievings // ♂ // Cylorygmus sp. / M. Hansen det. // HOLOTYPUS / Cylorygmus / repentinus sp.n. / des. F.Hebauer // Relictorygmus repentinus / (Hebauer, 2002) / det. M. Seidel 2017″. Paratype: female (SEMC): “R. S. Africa 17.xi.1993 / 34°27′S/20°24′E, leg. Uhlig / Cape Province: / De Hoop Nat. Res. / lake shore, reed sievings // ♀ // HOLOTYPUS / Cylorygmus / repentinus sp.n. / des. F.Hebauer // ex. F. Hebauer / Exchange 2008 //Relictorygmus repentinus / (Hebauer, 2002) / det. M. Seidel 2017″.
Additional material examined. 1♀ (NMPC): South Africa, Western Cape, Bot River near R43 bridge, 34°18.7′S 19°8.8′E, 17.-18.x.2013 (P. Bulirsch leg.).
Redescription of adult. 5.0–5.1 mm long, 2.8–2.9 mm wide (ca. 1.8× as long as wide), elongate oval, moderately convex (Figs 10A–B). Coloration. Dorsal coloration dark brown; margins of pronotum yellowish to light brown; ventral coloration dark brown, only hypomeron, epipleuron and legs light brown. Head. Eyes (Figs 10B–C) moderately large, interocular distance ca. 3.1× transverse diameter of eye. Mentum (Fig. 12K) with anterior margin arcuate mesally, moderately concave laterally, surface smooth and becoming sparsely setose towards lateral margins; gula (Fig. 12K) with median ridge strongly developed; gular sutures strongly concave. Prothorax. Prosternum (Fig. 12J) mesally with long setae, sparsely setose, surface of anterior marginal bead rather smooth; transverse ridge absent. Mesothorax. Mesoventrite (Figs 12L–M) with well-developed transverse groove posteromesally, reaching coxal cavities laterally, straight, surface posterior to the groove sparsely setose; elevated anterior part of mesoventrite subtriangular with weakly sinuate posterior margin. Metathorax (Fig. 12L). Raised median area of metaventrite with asetose anteriomedial area; anterior metaventral process wide, triangular, ca. 1.2× as long as maximum width. Legs. Ventral surface of profemora (Fig. 121) densely pubescent at base, sparsely pubescent at apex; mesofemora with moderately dense pubescence anterobasally, becoming sparser towards posterior margin and apex; metafemora sparsely pubescent. Male genitalia. Parameres (Fig. 9C) curved inwards, rounded at apices; median lobe ca. 0.5× as wide as parameres, rounded at apex, gonopore subapical.
Distribution. Only known from two localities in the southern extremes of Western Cape Province, South Africa (Fig. 12B).
Natural history. Specimens were collected in the middle of October and November, which correspond to the spring season in South Africa. The type specimens were obtained by sifting litter in reed stands at the shore of a nearly dried-up freshwater lake (Figs 13H–I). The additional specimen from the Bot River was collected by stepping its reed-covered muddy banks. Immature stages unknown.
Figure 12.
Details of adult morphology of Relictorygmus gen. nov., SEM micrographs. (A–H) Relictorygmus trevornoahi sp. nov., paratype: (A–C) femora in ventral view: (A) profemur; (B) mesofemur; (C) metafemur. (D) labrum in dorsal view; (E) prosternum; (F) head in ventral view; (G) meso- and metathorax in ventral view; (H) detail of mesoventrite. (I–M) R. repentinus (Hebauer, 2002), holotype: (I) profemur in ventral view; (J) median portion of prosternum; (K) head in ventral view; (L) meso- and metathorax in ventral view; (M) detail of mesoventrite.

Relictorygmus trevonoahi sp. nov.
Type material. Holotype: male (SAIM): “R. SOUTH AFRICA: W. Cape / 13.3 km SEE Stanford (wetland) / 34°27.85′S 19°35.75′E; 100 m / 4–5.xii.2015; Arriaga, Fikáček, Seidel & Vondráček lgt. RSA50 // isolated shallow/partly dried-up / pools in a small grassy wetland with / patches of Juncus and Phragmites: / in water and stepping/flooding the / mud/dried pools at sides”. Paratypes: 8 spec. (NMPC, BMNH, CDTB, ISAM, SEMC), 1 spec. (BMNH): same data as the holotype.
Description. 4.7–4.9 mm long, 2.7–2.9 mm wide (ca. 1.7× as long as wide), elongate oval, (holotype measurements: length = 4.8 mm, width = 2.9 mm, height = 1.8 mm); moderately convex (Figs 10D–E). Coloration. Dorsal coloration dark brown to black; margins of pronotum only slightly paler; ventral coloration including hypomeron, epipleuron and legs dark brown to black. Head. Eyes (Figs 10E–F) moderately large, interocular distance ca. 3.3× transverse diameter of eye. Mentum (Fig. 12F) with anterior margin blunt mesally, moderately concave laterally, surface becoming rugose and sparsely setose towards lateral margins; gula (Fig. 12F) with strongly developed median ridge; gular sutures strongly concave. Prothorax. Prosternum (Figs 11G, 12E) mesally with sparse moderately long setae on anterior half, posterior part bare; surface of anterior marginal bead rather smooth; anterior portion with weakly developed transverse ridge. Mesothorax. Mesoventrite (Figs 12G–H) with strongly developed transverse groove posteromesally, reaching coxal cavities laterally, angulate in shape, surface posterior to the groove bare; elevated anterior part of mesoventrite heart-shaped with deeply sinuate posterior margin. Metathorax. Raised median area of metaventrite with a setose anteriomedial area; anterior metaventral process wide, triangular, ca. 1.2× as long as maximum width. Legs. Ventral surface of profemora (Fig. 12A) sparsely pubescent at base and apex; with moderately dense pubescence anterobasally, becoming sparser towards posterior margin and apex; meso- and metafemora (Figs 12B–C) sparsely pubescent. Male genitalia. Parameres (Fig. 9E) with outer faces subparallel at basal half, then slightly converging apicad; inner face very weakly arcuate in apical third; apices widely rounded. Median lobe narrow, width at base ca. 2.0× the width at apical half, apex rounded, gonopore subapical.
Etymology. The species is dedicated to the South African comedian Trevor Noah whose performances were a welcomed entertainment to the first author.
Distribution. Only known from the type locality close to Stanford, Western Cape Province, South Africa (Fig. 13B).
Natural history. Specimens were collected at the beginning of December which corresponds to the end of the spring season in South Africa. The habitat (Figs 13F–G) was a marshy meadow with few small shallow water pools that were drying out. The nearly dried-up pools (without free water, but with the muddy bottom still wet) were flooded which resulted in specimens floating on the water surface. The following hydrophilid species were collected at the same locality as R. trevornoahi (but mostly in the pools still having some water): Allocotocerus simplex (Régimbart, 1906), Laccobius praecipuus Kuwert, 1890, Paracymus amplus Wooldridge, 1977, Limnoxenus sjostedti Knisch, 1924, Anacaena capensis Hebauer, 1999, Enochrus hartmanni Hebauer, 1998, Enoekrus cf. circumductus (Régimbart, 1905), Helochares dilutus (Erichson, 1843), Coelostoma austrine Mouchamps, 1958, Cercyon dieganus Régimbart, 1903 and Cercyon martialis Hebauer, 1997. An unidentified species of tiny Aulacochthebius Kuwert, 1887 (Coleoptera: Hydraenidae) was collected in the same microhabitat with the same method as R. trevornoahi, i.e. by floating the nearly dried-up pools.
Figure 13.
Distribution and habitats of Cylorygmus and Relictorygmus species. (A) known distribution in Chile; (B) known distribution in Western Cape, RSA (inset map shows position of maps A and B). (C–E) habitats of Cylorygmus lineatopunctatus Orchymont, 1933 in Sector Ocoa, PN La Campana, Chile: (C) small waterfall “La Cascada”; (D) water-soaked plant debris at base of the waterfall from which specimens were collected in 2013; (E) small pools in dried-up streambed in Quebrada Buitrera with debris from which specimens were collected in 2003. (F–G) type locality of Relictorygmus trevornoahi sp. nov.: (F) general view of the marshy meadow; (G) detail of dried-up pools with exposed bottom at side of main pools from which specimens were flooded. (H–I) type locality of Relictorygmus repentinus (Hebauer, 2002) at De Hoop village in De Hoop NR, Western Cape, RSA: (H) — M. Uhlig sifting litter in reed stands at the type locality; (I) general view of dried-up De Hoop vlei [= lake] north of De Hoop village, with the sampled reed stand). Photo credits: (C) — P. Kment; (D, F, G) — M. Fikáček; (E) — M. Thayer; (H, I) — M. Uhlig.

Discussion
Both genera treated in this paper, Cylorygmus and Relictorygmus gen. nov., are very similar to each other, and as such it is not surprising that they were not distinguished from each other until now. When examining their adult morphology, we discovered a number of differences (e.g. width of gula, presence/ absence of the stout setae on metafemora and abdominal ventrite I, presence/absence of stout setae on abdominal apex, sclerotization of abdominal tergites, general morphology of male genitalia, and different body shape) which indicated that the Chilean and South African species might not be closely related. On the other hand, characters so far considered as genusspecific in the Cylominae (morphology of mouthparts, the form of the mesoventrite) are very similar in both genera. A preliminary analysis of molecular data (Fikáček et al. in prep.) suggest that Cylorygmus and Relictorygmus are not closely related. This indicates that Cylorygmus and Relictorygmus may possess shared similarities that are plesiomorphies (characters inherited from ancestors) rather than apomorphies which would indicate their close relationships. The adult and larval synapomorphies of Cylominae are still unknown, but the assignment of both genera to the Cylominae was confirmed by our preliminary analysis, and that of Cylorygmus also by the analysis by McKenna et al. (2015).
The morphology of both adults and larvae seems to be congruent with the suggested plesiomorphic status of both genera, as both adults and larvae lack apparent derived structures and in most characters are in fact similar to genera of the subfamilies Hydrophilinae, Chaetarthriinae, Enochrinae and Acidocerinae. For example, larval nasale with five teeth and symmetrical mandibles with two large retinacular teeth as in Cylorygmus can be found in many genera of Hydrobiusini, in Sternoloplms Sober, 1834 (Hydrophilini) and many Anacaenini (Archangelsky 1997, Archangelsky and Fikáček 2004, Minoshima and Hayashi 2011a,b). In many of those (Hydrobiusini, Sternolophus, but also the laccobiine genus Tritonus Mulsant, 1844: Fikáček et al. 2017), the left lateralmost tooth of nasale is more distant from the other teeth, and the dorsal structure of mentum has a specific structure (i.e. it is covered by spinose cuticular projections except for two submedian bare areas: present in Hydrobiusini, Tritonus and Cylorygmus). All these characters shared with early branching representatives of different subfamilies indicate that the larval and adult morphology of Cylorygmus and Relictorygmus is plesiomorphic, and may stand close to the morphology of the supposed most recent common ancestor of the Hydrophilidae. Also, the habitat preferences of both genera (i.e. very humid but not submerged places at sides of water bodies) are shared with many groups of aquatic hydrophilids and may represent the ancestral state for the Hydrophilidae.
The separate generic status of Cylorygmus and Relictorygmus is more congruent with the distribution of both taxa. Besides South Africa and austral South America, Cylominae are only known from Australia, New Zealand, i.e. only from continents forming Gondwana during the Mesozoic. The break-up of Gondwana is hence supposed to play an important role in their current distribution. In contrast to Australia, New Zealand and South America, which kept some interconnections (or at least close proximity) until rather recently (ca. 50–30 millions of years ago (mya)), the African continent split from the others very early: it started to rift apart from southern South America ca. 135 mya and only northern South America stayed connected to Africa until ca. 110–95 mya (Sanmartín and Ronquist 2004). Bloom et al. (2014) estimated that the most recent common ancestor of Cylominae to have slived ca. 121.6 mya (HDP: 145–95 mya), i.e. at the time when Africa was already largely separated from South America, except in its northern part. If both Cylorygmus and Relictorygmus really represent ancient early branching clades of Cylominae as the preliminary molecular analyses suggest, available data would not allow to reject the hypothesis that ancestors of Cylorygmus and Relictorygmus originated by vicariant events corresponding to the break-up of Gondwana. On the other hand, the fact that the African Relictorygmus seems to stand closer to Australian and New Zealand genera, and Chilean Cylorygmus seems to form a lineage separated from all other cylomines does not fully correspond to the sequence of Gondwana break-up (this would predict South African clades as the most isolated ones). Further studies are necessary to understand this incongruence.
Conclusions
The limited set of characters applied by Hebauer (2002) in his description of the first African member of Cylominae resulted in the assumption of the trans-Atlantic disjunct distribution of the genus Cylorygmus. A detailed examination of the morphology of the Chilean and African species and the discovery of the third new species revealed that the Chilean and South African species are not closely related. This justifies the placement of the African species into a new genus, Relictorygmus gen. nov., breaking up an incorrectly assumed disjunct distribution of Cylorygmus. Understanding the presence of cylomine beetles in Africa will be crucial to understand the evolutionary and biogeographical history of these Gondwanan relicts. Molecular phylogenetics is necessary to be applied in future studies.
Author contribution
MS, EAV and MF performed the field work and accumulated additional museum material; MS performed the majority of morphological studies on adult beetles; YNM performed the morphological studies on larvae; MS prepared the first draft and photo documentation; all authors commented drafts of the paper at different stages and helped with completing the manuscript for submission.
Acknowledgements
We are grateful to Dominik Vondráček and Jiŕí Šmíd (National Museum, Prague) for their collaboration during the expedition to Western Cape province in 2015, to Manfred Uhlig (Museum für Naturkunde, Berlin) for sharing pictures of the type locality of R. repentinus with us, to Petr Bulirsch (Prague) for the donation of the only R. repentinus specimen known besides the type series, to Renzo Perissinotto (Nelson Mandela Metropolitan University) for his help with the logistics of our field trip and to the authorities of Cape-Nature for providing access to protected areas under their control. We are grateful to Víit Sýkora (Charles University, Prague) for providing his preliminary results on the Cylominae phytogeny. Alberto Ballerio (Brescia) is thanked for the discussion of the neotype issues. Furthermore, we thank all the curators listed in material and methods for loaning specimens to us. This work was supported by the European Union's Horizon 2020 research and innovation program under the Marie Skłdowska-Curie grant agreement No. 642241 to M. Seidel and E. Arriaga-Varela, by JSPS KAKENHI Grant Number JP17K15187 to Y. Minoshima, and by the Ministry of Culture of the Czech Republic (DKRVO 2018/13, National Museum, 00023272) to Martin Fikáček. The work of M. Seidel and E. Arriaga-Varela at the Department of Zoology, Charles University, Prague was partly supported by grant SVV 260 434 /2018. The Synthesys project, http://www.synthesys.info/ funded the stay of EA-V at the HNHM under the grant No. HU-TAF-6176.