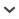The troglomorphic scorpion genus Troglotayosicus Lourenço, 1981, occurs in hypogean and epigean habitats in the Andean and Amazonian rainforests of Colombia and Ecuador. The phylogenetic relationships among the species of Troglotayosicus are currently unknown. In the present contribution, a new species, Troglotayosicus akaido, sp. nov., is described from specimens collected in the leaf litter of a primary rainforest in the Colombian Amazon, near the border with Peru, raising the number of species in the genus to seven. The new species represents the easternmost record of the genus and further extends its distribution into the Amazon. Its phylogenetic position was tested in an analysis of all species of the genus and two outgroup taxa, scored for 131 morphological characters (16 new and 115 legacy; 104 binary and 27 multistate) analyzed with maximum likelihood under the MK model. Troglotayosicus was recovered as monophyletic and composed of two main clades. The morphological survey revealed that the ventral macrosetae of the leg telotarsi of the type species, Troglotayosicus vachoni Lourenço, 1981, are simple, subspiniform macrosetae, irregularly distributed, but not arranged into clusters nor forming elongated clusters of setae/spinules, as previously suggested. A distribution map and key to the identification of the species of Troglotayosicus are provided. Further research, incorporating molecular data, is needed to understand the evolution and biogeographical history of this enigmatic scorpion genus.
INTRODUCTION
Troglotayosicus Lourenço, 1981, is among the most enigmatic scorpion genera in the Neotropics, due to its troglomorphism and rarity in biological collections. The genus was initially created by Lourenço (1981) to accommodate Troglotayosicus vachoni Lourenço, 1981, described from a single female collected in Los Tayos Cave (447 m), Ecuador, and temporarily assigned to the chactid subfamily Chactinae Pocock, 1893. Lourenço (1981) noted that the absence of median eyes in Troglotayosicus was shared with Belisarius Simon, 1879, an eyeless chactid genus endemic to the Pyrenees Mountains of France and Spain, but that the two genera could be recognized by the different trichobothrial patterns of the pedipalp chela and patella.
Stockwell (1989) was the first to observe morphological similarities between the pedipalp trichobothrial patterns of Troglotayosicus and another chactid, Superstitionia Stahnke, 1940. However, his phylogenetic hypothesis placed Troglotayosicus in a clade with Belisarius, to the exclusion of Superstitionia, preventing its placement in Superstitioniinae Stahnke, 1940 (as “Superstitioni-nae”). Stockwell (1989) therefore proposed creating two monotypic subfamilies Troglotayosicinae (as “Troglotayosinae”) and Belisariinae (as “Belisarinae”) of Chactidae Pocock, 1893, to accommodate these genera, but never published these changes to the classification.
Later, Stockwell (1992) reiterated that Troglotayosicus and Belisarius share troglomorphic characters such as the absence of median ocelli, but that Troglotayosicus exhibits a neobothriotaxic trichobothrial pattern similar to some Vaejovidae Thorell, 1876, whereas Belisarius exhibits an orthobothriotaxic trichobothrial pattern. Stockwell (1992) then elevated the chactid subfamily Superstitioniinae to the rank of family, Superstitioniidae Stahnke, 1940 (as “Superstitionidae”), and transferred Belisarius and Troglotayosicus to it, along with the troglomorphic genera previously assigned to the chactid subfamily Typhlochactinae Mitchell, 1971, a placement previously suggested by some authors (Vives, 1981; Dumont, 1986), but criticized by others (Lourenço, 1998; Sissom and Cokendolpher, 1998).
Lourenço (1998) essentially adopted Stockwell's (1989) unpublished proposal and erected family Troglotayosicidae Lourenço, 1998 (as “Troglotayosidae”), with subfamilies Belisariinae Lourenço, 1998 (as “Belisarinae”), and Troglotayosicinae Lourenço, 1998 (as “Troglotayosinae”), to accommodate Belisarius and Troglotayosicus, respectively. Lourenço (1998) argued that the disjunct, trans-Atlantic distribution observed in these genera could be explained by the same panbiogeographical “tracks” (Croizat, 1958) that explained the distribution of the disjunct scorpion family Iuridae Thorell, 1876. Subsequent authors (e.g., Fet and Sissom, 2000; Sissom, 2000a, 2000b; Soleglad and Fet, 2003a; Coddington et al., 2004) criticized the creation of Troglotayosicidae as weakly justified.
Next, Soleglad and Fet (2003a) synonymized Troglotayosicidae with Superstitioniidae, returning Troglotayosicus to Superstitioniidae and Belisarius to Chactidae. These authors recognized Superstitioniidae, in accordance with Stockwell (1992), as comprising subfamilies Superstitioniinae and Typhlochactinae based on four unique synapomorphies of the pedipalp trichobothria, pedipalp chela finger dentition, and metasomal segment V lateral carinae. Subfamily Superstitioniinae, comprising Superstitionia and Troglotayosicus, was supported by three homoplastic synapomorphies of the pedipalp chela and patella trichobothria and two unique synapomorphies of the pedipalp chela finger dentition and sternum. Soleglad and Fet (2003a: 28, 109, 141) scored the tarsal armature (char. 58)—ambiguously described for Troglotayosicus by Lourenço (1981)—as “elongated clusters of spinules” in Superstitionia and as “elongated clusters of setae/spinules” in Troglotayosicus. On the other hand, Coddington et al. (2004) disputed the putative relationship between Belisarius and Troglotayosicus, on the grounds that Belisarius appears to be more closely related to Euscorpiidae Laurie, 1896, than to Troglotayosicus.
FIGURE 1.
Known locality records of the species of Troglotayosicus Lourenço, 1981, in Colombia and Ecuador: (1) Troglotayosicus vachoni Lourenço, 1981; (2) Troglotayosicus humiculum Botero-Trujillo and Francke, 2009; (3) Troglotayosicus hirsutus Botero-Trujillo et al., 2012; (4) Troglotayosicus meijdeni Botero-Trujillo et al., 2017; (5) Troglotayosicus muranunkae Lourenço et al., 2020; (6) Troglotayosicus ballvei Botero-Trujillo et al., 2021; (7) Troglotayosicus akaido, sp. nov.

Prendini and Wheeler (2005) followed with a detailed critique of the analysis by Soleglad and Fet (2003a) and revalidated Troglotayosicidae. Prendini et al. (2010) subsequently presented a phylogenetic analysis of Typhlochactidae Mitchell, 1971, elevated to the rank of family by Vignoli and Prendini (2009), that confirmed the monophyly of Troglotayosicus, which by that time included a second species, Troglotayosicus humiculum Botero-Trujillo and Francke, 2009, based on a juvenile male collected with a Winkler trap in La Planada Natural Reserve, Ricaurte, Nariño Department, in the Colombian Andes (1885 m).
TABLE 1.
Known species of Troglotayosicus Lourenço, 1981, indicating countries, administrative areas and elevations of occurrence.

The species diversity of Troglotayosicus steadily increased in subsequent years (fig. 1, table 1). Soon after its description by Botero-Trujillo and Francke (2009), Ochoa et al. (2010) redescribed T. humiculum based on adult specimens from a second locality (1617 m) in Ricaurte, Nariño Department, and proposed new interpretations for the metasomal carinae and trichobothrial patterns of Troglotayosicus, as well as adding natural history observations on the habitat. Following these discoveries, Botero-Trujillo et al. (2012, 2017), using ultraviolet (UV) light to explore leaf litter, described Troglotayosicus hirsutus Botero-Trujillo et al., 2012, from Buesaco (1959 m), Nariño Department, and Troglotayosicus meijdeni Botero-Trujillo et al., 2017, from Rivera (880 m), Huila Department, both in the Colombian Andes. More recently, Sánchez-Vialas et al. (2020) described Troglotayosicus muranunkae Lourenço et al., 2020, from Nangaritza, Zamora-Chinchipe Province (940 m), and Botero-Trujillo et al. (2021) described Troglotayosicus ballvei Botero-Trujillo et al., 2021, from near Archidona, Napo Province (660 m), both collected in leaf-litter on the Amazonian slopes of the Andes in Ecuador. Several authors predicted the discovery of more endogean species of Troglotayosicus in the Andes and Amazonia (Botero-Trujillo et al., 2017; Sánchez-Vialas et al., 2020).
Despite a flurry of new species descriptions, the phylogenetic relationships among the species of Troglotayosicus remained unknown. In the present contribution, a new species of Troglotayosicus, Troglotayosicus akaido, sp. nov. (fig. 2A, B), is described from specimens collected in the leaf litter of a primary rainforest at 123 m in the Colombian Amazon, near the border with Peru, raising the number of species in the genus to seven (table 1). For the first time, the phylogenetic position of this new species is tested in an analysis of all species of the genus and two outgroup taxa, scored for 131 morphological characters, and analyzed with maximum likelihood under the MK model. A distribution map and key to the identification of the species of Troglotayosicus are also provided.
MATERIAL AND METHODS
Specimens of the new species were hand-collected using UV light during the night under a deep (ca. 30 cm) layer of leaf litter in primary rainforest, and preserved in 80% ethanol. Geographical coordinates of the collection locality were recorded with a portable Garmin Oregon® 600 GPS device.
The material examined for the study is housed in the arachnological collection of the Instituto de Ciencias Naturales (ICN), Universidad Nacional de Colombia, Bogotá; the American Museum of Natural History (AMNH), New York, including the Ambrose Monell Cryocollection (AMCC); the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia,” National Arachnological Collection (MACN-Ar), Buenos Aires, Argentina; the Museo Javeriano de Historia Natural “Lorenzo Uribe S. J.,” Pontificia Universidad Javeriana (MPUJ), Bogotá, Colombia; and the Museo de Zoología (QCAZ), Pontificia Universidad Católica del Ecuador, Quito. In addition to the type material of the new species, another six ingroup species and two outgroup species were examined for the study (appendix 1). The holotype and only known specimen of T. muranunkae was unavailable for examination hence characters for this species were scored from the original description (Sánchez-Vialas et al., 2020) and unpublished images provided by J. Blasco-Arostegui. Characters of the adults of T. hirsutus were also scored from the original description (Botero-Trujillo et al., 2012).
Specimens were examined with a Nikon SMZ1500 trinocular stereomicroscope fitted with an ocular micrometer. Digital images were taken using a Nikon D3300 DSRL camera and stacked using ZereneStacker ( https://zerenesystems.com). Habitus images were prepared using a Nikon 65 mm lens with a Godox MF12 dual macro flash and the camera controlled by a StackShot macro rail ( https://cognisys-inc.com). Morphological images were prepared by connecting the camera to the stereomicroscope ocular lens with a DSLR adapter. Camera settings and live view were controlled using qDslrDashboard 3.6.7 ( https://dslrdashboard.info). Images were edited with GIMP 2.10 ( http://www.gimp.org) and plates prepared with INKSCAPE 1.3 ( http://www.inkscape.org).
The generic diagnosis was modified from Botero-Trujillo et al. (2021). The description style mostly follows Ochoa et al. (2010) and Botero-Trujillo et al. (2017). General morphological terminology follows Stahnke (1970) and Sissom et al. (1990) except for cheliceral dentition (Vachon, 1963), replacing internal with ventral and external with dorsal; lateral ocelli (Loria and Prendini, 2014); sternum (Soleglad and Fet, 2003b); pedipalpal and metasomal carination (Prendini, 2000, 2003a); and telotarsal ventral macrosetal counts (Francke, 1977). Pedipalp trichobothrial pattern follows two proposals simultaneously, the “placeholder” terminology of Prendini et al. (2010) and a modified version of Vachon's (1974) terminology, reinterpreted based on the trichobothrial pattern of Typhlochactas Mitchell, 1971, by Prendini and Wheeler (2005) and Vignoli and Prendini (2009).
The following abbreviations are used: ocelli: posterodorsal minor ocellus (PDMi); posterolateral major ocellus (PLMa); pedipalp and leg carinae and processes: digital (DI), dorsomarginal (DMA), dorsal patellar process (DPP), dorsal secondary (DS), prodorsal (PD), promedian (PM), proventral (PV), retrodorsal (RD), retrolateral (RL), retroventral (RV); trichobothrial pattern: dorsal (D, d), dorsobasal (Db, db), dorsomedian (dm), dorsal subbasal (dsb), dorsal subterminal (dst), external (e), external basal (Eb, eb), external subbasal (Esb, esb), externomedian (em), external subterminal (Est, est), external terminal (Et, et), internal (i), internal basal (ib), median (m), ventral (V); opisthosomal carinae: dorsolateral (DL), lateral inframedian (LIM), lateral supramedian (LSM), median lateral (ML), ventrolateral (VL), ventromedian (VM), ventrosubmedian (VSM); hemispermatophore: external lobe (EL), internal lobe (IL); measurements: length (L), height (H), width (W).
The distribution map was prepared using R 4.3.1( https://www.r-project.org), with elevation data obtained from the elevatr package (Hollister et al., 2023), country and river spatial data from giscoR (Hernangómez, 2023) and rnaturalearth (Massicotte and South, 2023), and plotting performed using the ggplot2 (Wickham, 2011, 2016) and ggspatial (Dunnington et al., 2023) packages. Geographical coordinates of published locality records were extracted from previous publications (Lourenço, 1981; Botero-Trujillo and Francke, 2009; Botero-Trujillo et al., 2012, 2017, 2021; Sánchez-Vialas et al., 2020).
A matrix of 131 morphological characters (16 new and 115 legacy; 104 binary and 27 multistate) was scored for nine species (seven from the ingroup) of three genera (appendices 2, 3), with Superstitionia donensis Stahnke, 1940, set as root a posteriori, following Prendini et al. (2010). Sixteen characters were rescored for some terminals (e.g., S. donensis, T. humiculum, and T. vachoni) based on the examination of available materials.
Tree search was conducted using IQTREE with the maximum likelihood criterion and the MK model (Minh et al., 2020). Ultrafast bootstrap was calculated after 1000 replications. Morphological synapomorphies were unambiguously optimized using WinClada (Nixon, 2002). Tree files were edited with Figtree 1.4.4 ( https://github.com/rambaut/figtree) and INKSCAPE 1.1 ( http://www.inkscape.org).
RESULTS
Phylogenetic Analysis
The log-likelihood of the phylogenetic tree was -527.3571 (fig. 3). Troglotayosicus was recovered as monophyletic. Despite the low bootstrap support values (bts = 37), the genus was supported by 21 unique synapomorphies (appendix 2) pertaining to the carapace (chars. 6, 12), pedipalp chela dentition (chars. 19, 20), pedipalp chela ornamentation (chars. 22, 23), pedipalp femur trichobothria (chars. 32, 33, 34), pedipalp patella trichobothria (chars. 42, 44, 45, 46, 50), pedipalp chela trichobothria (chars. 69, 76, 77), pectines (char. 104), sternites (char. 112), and metasoma (chars. 118, 119).
Two major clades were recovered within Troglotayosicus. One major clade, comprising T. vachoni, placed sister to a smaller clade containing T. ballvei and T. humiculum, was supported by one homoplastic synapomorphy (char. 123) and received a low bootstrap value (bts = 25). The clade comprising T. ballvei and T. humiculum, supported by one homoplastic synapomorphy (char. 107), also received a low bootstrap value (bts = 32). The other major clade of Troglotayosicus, comprising the remaining four species of the genus (T. hirsutus, T. muranunkae, T. akaido, T. meijdeni), was supported by one unique synapomorphy (char. 127) and received a low bootstrap value (bts = 36). This clade contained T. muranunkae, placed sister to a clade comprising T. akaido and T. meijdeni, which was supported by one homoplastic synapomorphy (char. 90) and received a low bootstrap value (bts = 39). The clade comprising T. akaido and T. meijdeni, supported by two homoplastic synapomorphies (chars. 81, 125) and one unique synapomorphy (char. 126), received a high bootstrap value (bts = 89).
FIGURE 3.
Phylogeny of Troglotayosicus Lourenço, 1981, based on maximum likelihood analysis of 131 morphological characters (appendices 2, 3). Unambiguous character states optimized and plotted on branches. Black circles indicate uniquely derived apomorphic states, white circles parallel derivations of apomorphic states. Numbers above branches correspond to character numbers; numbers below branches to character states (appendix 3); numbers at nodes indicate ultrafast bootstrap values.

Based on an examination of adult specimens of T. vachoni, the distribution of the ventral macrosetae of the telotarsi was confirmed to be irregular in this species (char. 94). However, the suggestion that these structures might be spinules or setae/spinule clusters (Lourenço, 1981, 1998; Soleglad and Fet, 2003a) was refuted. The irregular distribution of the ventral macrosetae of the telotarsi was found to be a homoplastic synapomorphy for T. vachoni and T. muranunkae.
The clades of Troglotayosicus recovered in the present analysis do not exhibit an obvious pattern consistent with the biogeographical regions presented by Morrone et al. (2022). In the clade comprising T. ballvei, T. humiculum, and T. vachoni, it is noteworthy that T. ballvei occurs in the Napo Province and T. vachoni in the Páramo Province, both located in the Andean-Amazon foothills, whereas T. humiculum occurs in the Cauca Province of the Andean-Pacific foothills. On the other hand, in the clade comprising the remaining four species of the genus, it is remarkable that T. hirsutus occurs in the Cauca Province and T. meijdeni in the Magdalena Province, both located in the Colombian Andes, whereas T. muranunkae occurs in the Páramo Province of the Andean-Amazonian foothills, and T. akaido in the Imerí Province of the Amazonian lowlands.
SYSTEMATICS
Order Scorpiones C. L. Koch, 1837
Family Troglotayosicidae Lourenço, 1998
Genus Troglotayosicus Lourenço, 1981
Figures 1–14
Troglotayosicus Lourenço, 1981: 650, 651, type species by original designation: Troglotayosicus vachoni Lourenço, 1981.
Diagnosis: Cheliceral movable finger with well-developed serrula ventrally. Carapace with anteromedian epistome weak to moderate; median ocelli absent; lateral ocelli pattern Type 2B, with two ocelli (PLMa and PDMi) present and four ocelli (ALMa, MLMa, ADMi, and PLMi) absent; lateral eyespot situated ventral to lateral ocelli or absent. Sternum subpentagonal. Pedipalp chela with DS, DI, RL, RV, PV, DMA, and PD carinae identified by subtle differences in angles between adjacent surfaces, smooth or with DS, DMA, and PD granular; median denticle row of fixed finger comprising six oblique primary subrows of denticles, terminal denticle larger than others and hooklike; median denticle row of movable finger comprising seven oblique primary subrows of denticles, terminal denticle larger and hooklike, accommodated in subdistal diastema of fixed finger; pedipalp fingertips interlocking unevenly when closed such that movable finger displaced retrolaterally, relative to fixed finger. Pedipalps with three trichobothria on femur, 19 on patella, and 26 on chela. Legs without tibial spur; basitarsi with rows of comblike macrosetae distally. Posttergites I–VI finely granular or predominantly smooth, acarinate; VII finely granular, posterior half with scattered granules in position of DSM carinae and with DL carinae terminating in tubercle comprising four or five granules. Mesosoma with rounded book lung spiracles; male and female pectines each with 7 or 8 teeth, without fulcra. Metasoma with VL and VSM carinae absent on segments I and II, and occasionally also on III and IV; LIM carinae present on segments I–III, present or absent on IV, and sometimes indistinct on V; segment V with paired DL, LSM, ML, VL, and VSM carinae, and single VM carina, comprising discontinuous granules not arranged into distinct rows. Telson vesicle without subaculear tubercle; aculeus stout basally, slightly tapering distally. Hemispermatophore lamelliform; lamina with elongate apex, without crests, articular flexure present; basal portion and foot well developed, similar in length to lamina; capsule simple, with ectal and ental lobes; IL more pronounced than EL, with sclerotized ectal spurlike projection.
Included Species: Troglotayosicus akaido, sp. nov.; Troglotayosicus ballvei Botero-Trujillo et al., 2021; Troglotayosicus hirsutus Botero-Trujillo et al., 2012; Troglotayosicus humiculum Botero-Trujillo and Francke, 2009; Troglotayosicus meijdeni Botero-Trujillo et al., 2017; Troglotayosicus muranunkae Lourenço et al., 2020; Troglotayosicus vachoni Lourenço, 1981.
Distribution: Known only from Colombia and Ecuador, where it has been recorded in the Andean and Amazonian regions at elevations ranging from 123 to 1959 m.
Key to Identification of the Species of Troglotayosicus Lourenço, 1981
1. Lateral eyespot absent (fig. 5C, F); pedipalp chela trichobothrium e8 (Et4) situated distal to trichobothrium m4 (dsb) (figs. 8C, D, 9D) and trichobothrium i3 (it) situated midway between trichobothrium i4 (ib) and base of fixed finger (figs. 8G, H, 9H) T. akaido, sp. nov.
– Lateral eyespot present (Botero-Trujillo et al., 2021: 7, fig. 4B); pedipalp chela trichobothrium e8 (Et4) situated proximal to trichobothrium m4 (dsb) (Botero-Trujillo et al., 2021: 12, 13, figs. 8B, 9B) and trichobothrium i3 (it) situated closer to base of fixed finger than to trichobothrium i4 (ib) (Botero-Trujillo et al., 2021: 12, 13, figs. 8B, 9D) 2
2. Telson LIM, VSM and VL carinae present, complete (Ochoa et al., 2010: 13, fig. 10D–F) 3
– Telson LIM carinae absent; VSM and VL carinae vestigial, reduced to few granules at anteroventral margin of segment (Botero-Trujillo et al., 2012: 73, figs. 23, 24) 5
3. Pedipalp patella trichobothrium V1 situated distal to trichobothrium esb1 (Botero-Trujillo et al., 2021: 11, fig. 7C–H); leg III basitarsus, proventral row of macrosetae and leg IV basitarsus, retroventral row of macrosetae present T. ballvei
– Pedipalp patella trichobothrium V1 aligned with trichobothrium esb1 (Ochoa et al., 2010: 10, fig. 7B, D, F); leg III basitarsus, proventral row of macrosetae and leg IV basitarsus, retroventral row of macrosetae absent 4
4. Leg III basitarsus, retroventral row of macrosetae present (obsolete, represented by one or two macrosetae), telotarsus ventral macrosetae arranged into pair of distinct ventrosubmedian rows; metasomal segment III with VSM and VL carinae sparsely granular (Ochoa et al., 2010: 12, 13, figs. 9C, 10C) T. humiculum
– Leg III basitarsus, retroventral row of macrosetae absent, telotarsus ventral macrosetae irregularly arranged; metasomal segment III ventral surface acarinate, smooth T. vachoni
5. Lateral eyespot similar in size to PDMi ocellus (Botero-Trujillo et al., 2017: 574, fig. 3B); pedipalp patella trichobothrium V1 situated distal to trichobothrium esb1 (Botero-Trujillo et al., 2017: 578, fig. 7A–C); metasomal segment V with VM carina restricted to posterior two-thirds of segment (Botero-Trujillo et al., 2017: 579, fig. 8E) T. meijdeni
– Lateral eyespot smaller than PDMi ocellus; pedipalp patella trichobothrium V1 aligned with trichobothrium esb1 (Botero-Trujillo et al., 2012: 71, figs. 19–21); metasomal segment V with VM carina complete 6
6. Surfaces densely setose (Botero-Trujillo et al., 2012: 71, figs. 8–11); pedipalp chela trichobothrium D1 (Db) situated proximally on manus, slightly distal to trichobothrium Eb3 (Botero-Trujillo et al., 2012: 71, fig. 14); leg I basitarsus, retroventral row of macrosetae present T. hirsutus
– Surfaces moderately setose (Sánchez-Vialas et al., 2020: 615, fig. 2A, B); pedipalp chela trichobothrium D1 (Db) situated submedially on manus, approximately midway between trichobothria Eb3 and d8 (db) (Sánchez-Vialas et al., 2020: 616, fig. 5A); leg I basitarsus, retroventral row of macrosetae absent T. muranunkae
Troglotayosicus akaido, sp. nov.
Figures 1, 2, 3, 4A–D, 5A–F, 6A, B, 7A–H, 8A–H, 9A–H, 10A, B, 11A–F, 12A–F, 13A, B, 14A–D; tables 1, 2
Type Material: COLOMBIA: Amazonas Department: El Encanto Municipality: Río Caraparaná, Comunidad de San Rafael, 01°41′10.5″S 73°13′18.7″W, 123 m, 23–24.iv.2022, D. Luna and G. Cobette, holotype ♂, 2 ♂, 2 ♀, 2 subad. ♀, 1 juv. paratypes (ICN).
Diagnosis: Troglotayosicus akaido, sp. nov., shares with T. ballvei, T. hirsutus, T. humiculum, and T. meijdeni the regular distribution of ventral macrosetae on the telotarsi into two ventrosubmedian rows, differing in this respect from T. muranunkae and T. vachoni, in which the ventral macrosetae on the telotarsi are irregularly distributed. The tegument is moderately setose in T. akaido (fig. 4A–D), T. ballvei, T. humiculum, T. meijdeni, T. muranunkae, and T. vachoni, unlike in T. hirsutus, in which the tegument is densely setose (Botero-Trujillo et al., 2012: 69, figs. 8–11). Trichobothrium D1 (Db) is situated proximally on the pedipalp chela manus, and slightly distal to trichobothrium Eb3 in T. akaido (fig. 9B), T. ballvei, T. humiculum, T. hirsutus, T. meijdeni, and T. vachoni, but situated submedially on the manus, between trichobothria Eb3 and d8 (db) in T. muranunkae (Sánchez-Vialas et al., 2020: 616, fig. 5A). Trichobothrium i3 (it) is situated midway between trichobothrium i4 (ib) and the base of the chela fixed finger in T. akaido (fig. 9H), but closer to the base of the fixed finger than to trichobothrium i4 (ib) in all other species of the genus. The pedipalp chela exhibits distinct sexual dimorphism, with a more incrassate manus in the adult males of T. akaido (fig. 8A, C, E, G), T. hirsutus, T. humiculum, and T. vachoni, whereas the chela shape is similar in the adults of both sexes in T. ballvei (Botero-Trujillo et al., 2021: 12, fig. 8A–D) (the adult male is unknown in T. meijdeni and T. muranunkae). The VSM and VL carinae of metasomal segment IV are incomplete and reduced to scattered granules in the anterior half of the ventral surface in T. akaido (fig. 11C, F), T. ballvei, and T. vachoni, whereas these carinae are complete in T. humiculum (Ochoa et al., 2010: 12, 13, figs. 9C, 10C), and absent in T. hirsutus (Botero-Trujillo et al., 2012: 73, fig. 24), T. meijdeni, and T. muranunkae. The VSM and VL carinae of metasomal segment V are complete in T. akaido (fig. 12C, F), T. ballvei, T. humiculum, and T. vachoni, whereas these carinae are partial, reduced to the posterior two-thirds of the segment in T. hirsutus (Botero-Trujillo et al., 2012: 73, fig. 24), T. meijdeni, and T. muranunkae.
Troglotayosicus akaido differs further in the absence of a lateral eyespot (fig. 5C, F) and in the position of trichobothrium e8 (Et4), which is situated distal to trichobothrium m4 (dsb) on the pedipalp chela manus (fig. 9D); in all other species of the genus, the lateral eyespot is present and trichobothrium e8 (Et4) is situated proximal to trichobothrium m4 (dsb).
Etymology: Named after the word akaìdo, which means “scorpion” in the Huitoto Murui language. The Murui people live in the vicinity of the type locality.
Description: Based on the holotype ♂ (ICN) (fig. 2) and paratype ♀ (ICN). Total length: male, 19.53 mm; female 18.44 mm (table 2).
Coloration: General pattern (in 70% ethanol) reddish brown (fig. 4A–D). Carapace (fig. 5A–C), pedipalps, and tergites uniformly reddish brown, with carapacial sulci less pigmented and lateral ocelli surrounded by black spots. Chelicerae, coxosternal region, legs, and sternites uniformly light reddish brown. Metasomal segments dark reddish brown, becoming progressively darker posteriorly. Telson reddish brown; aculeus black.
Chelicerae: Manus dorsal and retrolateral surfaces smooth, sparsely covered with setae; prolateral and ventral surfaces densely covered with setae. Fixed finger with four dorsal teeth (distal, subdistal, median, and basal); median and basal teeth separate, not fused into bicusp; teeth progressively increasing in size distally. Movable finger with five dorsal teeth (prodistal, two small subdistal, median, and basal); teeth progressively increasing in size distally, except for subdistal teeth that are smaller than median and basal teeth; prodistal and retrodistal teeth not opposable; ventral surface with long, well-developed serrula, and vestigial retrobasal denticle.
Carapace: Surfaces sparsely covered with coarse granules and few fine granules (fig. 5A–F); anterior margin with round, slightly protruding epistome; lateral ocular carinae, central lateral carinae, and posterior median carinae distinct, other carinae absent; lateral ocular sulci, central transverse sulcus, posterior lateral sulci, and posterior transverse sulcus distinct; median ocelli absent; two pairs of lateral ocelli (PDMi and PLMa) (Type 2B); eyespot absent.
Sternum: Shape subpentagonal with rounded apex (fig. 6A, B); posterior depression shallow, sparsely setose on median surface and with many microsetae along anterior margin.
Pectines: Pectinal plate sexually dimorphic (fig. 6A, B), 1.8× wider than long and square (♂) or 2.2× wider than long and rectangular (♀); densely (♂) or sparsely (♀) setose. Pectinal tooth count 7/7 (♂, ♀), teeth longer and wider in ♂; teeth, medial and marginal lamellae densely setose.
Pedipalps: Femur with three (♀) or five (♂) carinae (fig. 7A, B); PD, RD, and PM carinae crenulate and complete; PV and RV carinae restricted to proximal third (♂) or absent (♀); dorsomedian and proventral surfaces sparsely and coarsely granular; prodorsal surface sparsely and finely granular; retrodorsal and retroventral surfaces smooth; ventral surface smooth (♀) or sparsely and coarsely granular in proximal third (♂). Patella with two carinae (fig. 7C–H); PD and PV carinae crenulate and restricted to proximal third; DPP composed of three or four coarse granules; all surfaces smooth. Chela manus (tibia) broader in ♂ (L/W, 2.63; L/H, 2.28) than in ♀ (L/W, 2.72; L/H, 2.29) (fig. 8A–H). Manus with seven obsolete carinae; DS, DI, RL, RV, and PV carinae smooth; DMA carina granular along entire length (♂) or only in distal half (♀); PD carina granular; intercarinal surfaces smooth and setose along carinae. Fixed and movable fingers without lobes or depressions; prolateral and retrolateral surfaces densely setose; fixed finger with six oblique, slightly imbricate rows of denticles, flanked by six prolateral and five retrolateral denticles, terminal denticle enlarged; movable finger with seven oblique, slightly imbricate rows of denticles, flanked by seven prolateral and six retrolateral denticles, terminal denticle enlarged.
Trichobothria: Femur with three trichobothria (fig. 9A): one each on retrolateral (d5 [e]), dorsal (d3 [d]), and prolateral (i1 [i]) surfaces. Patella with 19 trichobothria (fig. 9C, E, G): two on ventral surface (V1, V3 [V2]); 14 on retrolateral surface (et9 [et1], et3 [et2], V4 [et3], est1 [est], em1, et6 [em2], em4 [em3], esb1, esb4 [esb2], eb2 [eb1], eb3 [eb2], eb4 [eb3], eb5 [eb4], eb9 [eb5]), including two petite (est1 [est], esb4 [esb2]) and one accessory (em4 [em3]); two on dorsal surface (d1, d2); and one on prolateral surface (i1 [i]). Chela manus with 26 trichobothria (fig. 9B, D, F, H): 15 on manus, four on ventral surface (V1, i5 [V2], V3, V5 [V4]), ten on retrolateral surface (Et1, Et2, Est4 [Et3], e8 [Et4], Est6 [Et5], Est3 [Est], Esb1 [Esb], Eb1–Eb3), and one on dorsal surface (D1 [Db]); and 11 on fixed finger, four on retrolateral surface (e3 [et], e5 [est], e6 [esb], Et6 [eb]), five on dorsal surface (d4 [dt], m2 [dst], m3 [dm], m4 [dsb], d8 [db]), and two on prolateral surface (i3 [it], i4 [ib]).
Tergites: Pretergites smooth; posttergites smooth in anterolateral half, granular posteromedially and posterolaterally; posttergite VII with lateral carina granular, terminating in enlarged granule, median and submedian surfaces coarsely granular.
Sternites: Surfaces smooth; ventral surface sparsely setose, lateral and posterior margins densely setose (fig. 10A, B). Book lung spiracles subcircular.
Legs: Legs I–IV with carinae more developed on III and IV than on I and II. Femur tricarinate (less pronounced in ♀), RV and PM carinae continuous, granular, PD carina restricted to proximal third, fused with RV carina medially. Tibia sparsely setose, tibial spurs absent. Basitarsi of legs I–IV setose, each with dorsal and ventral rows of comblike macrosetae distally; dorsal retrolateral comb present, well developed on legs I–IV, but shorter on I and II; ventral prolateral comb present on I–III, absent on IV; prolateral pedal spurs present; retrolateral pedal spurs vestigial. Telotarsi I–IV, dorsomedian lobe with one macroseta; ventral surface without spinules, subspiniform macrosetae regularly arranged into pair of parallel ventrosubmedian rows. Telotarsi, counts of macrosetae in proventral and retroventral rows of sinistral (S) and dextral (D) legs I–IV (proventral S–D/retroventral S–D): ♂, 6–5/7–7, 6–6/8–6, 7–?/9–?, 9–9/8–8; ♀, 7–6/5–4, 6–6/9–7, 7–6/8–8, ?–?/?–?; ungues short, symmetric.
Metasoma: Segments I–V progressively increasing in length (figs. 11A–F, 12A–F). Segments I–V, dorsal intercarinal surfaces smooth; lateral intercarinal surfaces sparsely granular; ventral intercarinal surfaces smooth on I–III, coarsely granular in posterior half of IV. Segment V, dorsal intercarinal surface smooth; lateral surfaces entirely coarsely and densely granular, ventral surface coarsely and densely granular in posterior half. DL carinae complete, serrato-crenulate, converging posteriorly on segments I–IV; complete, crenulate on V; LSM carinae obsolete, partial, each comprising row of granules between DL and ML carinae, in posterior half of segments I–IV; ML carinae complete, serrato-crenulate on segments I–IV, complete but obscured by coarse granulation on V; LIM carinae obsolete, comprising isolated granules, complete on segment I, restricted to posterior two-thirds on II–IV and to anterior half on V; VL and VSM carinae absent on segments I–III, consisting of discontinuous, coarse granules in medial third of IV; VSM and VM carinae absent on segments I–IV, present but obscured by coarse granulation on V.
Telson: Vesicle suboval, elongate (fig. 13A, B), narrower in ♂ (L/H, 3.63) than in ♀ (L/W, 2.93) (table 2); anterodorsal lateral lobes present; dorsal and dorsolateral surfaces smooth; ventral and ventrolateral surfaces smooth (♂) or with three sparsely granular rows (obsolete VM and VSM carinae) anteriorly (♀) and numerous macro- and microsetae. Aculeus long, stout basally.
Hemispermatophore: Shape lamelliform (fig. 14A–D). Lamina translucent and weakly sclerotized, broad proximally and medially, and progressively tapering distally; crests absent; apex elongate, strongly recurved. Capsular region simple; external lobe (EL) medium sized, inclined ventrally; internal lobe (IL) medium sized, thick, with dorsal process, inclined entally; IL cylindrical, elongate, slightly larger than EL, with sclerotized spurlike projection directed ventrally; median lobe absent. Foot flexure present, trunk well developed, weakly sclerotized, twice as broad as basal portion of lamina.
Variation: Total length: ♂, 15.03–19.53 mm (n = 3; table 2), ♀, 16.17–18.44 mm (n = 2). Telotarsi ventral macrosetal rows: 6 or 7 prolateral and 5 to 9 retroventral macrosetae on leg I, 6 to 8 proventral and 6 to 8 retroventral on leg II, 7 to 9 proventral and 9 or 10 retroventral on leg III, and 7 to 9 proventral and 8 to 10 retroventral on leg IV (♂) (n = 3; table 2); 5 to 7 prolateral and 4 to 6 retroventral macrosetae on leg I, 6 to 9 proventral and 5 to 7 retroventral on leg II, 7 to 9 proventral and 6 to 8 retroventral on leg III, and 7 or 8 proventral and 9 or 10 retroventral on leg IV (♀) (n = 2; table 2).
Distribution: Known only from the type locality, Río Caraparaná, in the El Encanto Municipality of the Amazonas Department of Colombia (fig. 1).
Natural history: The specimens of T. akaido were collected in a secondary tropical terra firme rainforest at elevations of 123 to 150 m. Adults were found under a deep layer of leaf litter, 10 to 25 cm below the surface. Some juveniles were found closer to the surface in the leaf litter. One juvenile was collected in a disturbed forest near the Chagra ecosystem. This species was sympatric with four other scorpion species, Ananteris sp., Chactopsis sp., Tityus bastosi Lourenço, 1984, and Tityus sp.
Scorpionism: The second author was stung on the right thumb during collection of the male holotype. Intense pain, without paralysis or thermal sensation, was experienced for 30 minutes after envenomation, followed by local redness and inflammation. The affected area developed hyperkeratosis after three or four days. No other symptoms were experienced.
Additional Material Examined: COLOMBIA: Amazonas Department: El Encanto Municipality: Río Caraparaná, Comunidad de San Rafael, 01°41′10.5″S 73°13′18.7″W, 150 m, 23.iv.2022, D. Luna, manual collecting using UV light, inside leaf litter, 30 cm deep, 2 juv. (AMCC [LP 18087, 18093]).
FIGURE 4.
Troglotayosicus akaido, sp. nov., habitus, dorsal (A, C) and ventral (B, D) aspects. A, B. Holotype ♂ (ICN). C, D. Paratype ♀ (ICN). Scale bars: 10 mm.

TABLE 2.
Measurements (mm) of type material of Troglotayosicus akaido, sp. nov., deposited in the Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá. * Sum of lengths of femur, patella, and chela.

FIGURE 5.
Troglotayosicus akaido, sp. nov., carapace, dorsal aspect, under white light (A, B) and UV light (D, E) and lateral ocelli under white light (C) and UV light (F). A, D. Holotype ♂ (ICN). B, C, E, F. Paratype ♀(ICN). Scale bars: 2 mm (A, B, D, E); 0.6 mm (C, F).

FIGURE 6.
Troglotayosicus akaido, sp. nov., coxosternal region and pectines, ventral aspect. A. Holotype ♂(ICN). B. Paratype ♀ (ICN). Scale bars: 1.5 mm.

FIGURE 7.
Troglotayosicus akaido, sp. nov., dextral pedipalp femur, dorsal aspect (A, B), and patella, dorsal (C, D), retrolateral (E, F), and ventral (G, H) aspects. A, C, E, G. Holotype ♂ (ICN). B, D, F, H. Paratype ♀ (ICN). Scale bar: 0.7 mm.

FIGURE 8.
Troglotayosicus akaido, sp. nov., dextral pedipalp chela (tibia), dorsal (A, B), retrolateral (C, D), ventral (E, F), and prolateral (G, H) aspects. A, C, E, G. Holotype ♂ (ICN). B, D, F, H. Paratype ♀ (ICN). Scale bar: 1.5 mm.

FIGURE 9.
Troglotayosicus akaido, sp. nov., trichobothrial pattern of dextral pedipalp femur (A), chela (tibia) (B, D, F, H), and patella (C, E, G), dorsal (A, B, C), retrolateral (D, E), ventral (G, F), and prolateral (H) aspects. A–H. Holotype ♂ (ICN). Trichobothrial nomenclature outside parentheses follows Prendini et al. (2010), whereas nomenclature inside parentheses follows a modified version of Vachon's (1974) terminology, reinterpreted based on the trichobothrial pattern of Typhlochactas Mitchell, 1971, by Prendini and Wheeler (2005) and Vignoli and Prendini (2009). Scale bar: 1 mm.

FIGURE 10.
Troglotayosicus akaido, sp. nov., sternites IV–VII, ventral aspect. A. Holotype ♂ (ICN). B. Paratype ♀ (ICN). Scale bars: 2.5 mm.

FIGURE 11.
Troglotayosicus akaido, sp. nov., metasomal segments I–IV, dorsal (A, D), lateral (B, E) and ventral (C, F) aspects. A–C. Holotype ♂ (ICN). D–F. Paratype ♀ (ICN). Scale bar: 1.5 mm.

ON THE MORPHOLOGY OF TROGLOTAYOSICUS VACHONI
Prior to the present contribution, only the original author of the species (Lourenço, 1981) had examined a specimen of T. vachoni. Three attempts by the last author to examine the holotype of T. vachoni during visits to the Muséum National d'Histoire Naturelle, Paris, in 2004, 2005 and 2019 met without success. Consequently, the characters scored for T. vachoni in all previous phylogenetic analyses (Stockwell, 1989; Soleglad and Fet, 2003a; Vignoli and Prendini, 2009; Prendini et al., 2010; Santibáñez-López et al., 2014b) were based entirely on the somewhat ambiguous original description of the holotype (Lourenço, 1981). The availability of new material, including the first adult male specimens, of T. vachoni has allowed several misconceptions in the literature to be rectified. For example, the ventral macrosetae of the leg telotarsi are simple, subspiniform macrosetae, irregularly distributed, but not arranged into clusters as stated by Lourenço (1981) nor forming elongated clusters of setae/spinules as suggested by Soleglad and Fet (2003a). The ventral setation of the leg telotarsi has often been used as a diagnostic character at the genus and species levels in families such as Bothriuridae Simon, 1880, Chactidae, Diplocentridae Karsch, 1880, Hormuridae Laurie, 1896 and Vaejovidae (Lourenço, 2002a, 2000b; Prendini, 2000, 2003b; McWest, 2009). However, the phylogenetic analysis of Troglotayosicidae presented herein does not suggest that T. vachoni represents a different genus, distinct from the other species of Troglotayosicus, as this character appears to be a homoplastic synapomorphy with T. muranunkae, assuming the description of the tarsal armature of the latter species, described as “telotarsi not arranged in rows,” is accurate (Sánchez-Vialas et al., 2020: 616). Further studies incorporating additional specimens of T. muranunkae and molecular data for all species of Troglotayosicus are needed to understand the evolution and biogeographical history of this enigmatic scorpion genus.
ACKNOWLEDGMENTS
We are grateful to Agustina Flores (El Encanto community) and Gaspar Cobette (San Rafael community) for participating in the fieldwork in Colombia, and Andrés Alberto Barona Colmenares for logistical and administrative support throughout the expedition; Gregorio Gaique, Venedicto, and Gaspar Cobette for help with identifying arachnid names in the Bue language; Catalina Giraldo for providing photographs of the live habitus of the new species; the SINCHI institute (Instituto Amazónico de Investigaciones Científicas, Colombia) for support and collaboration; Javier Blasco-Aróstegui (Universidade de Lisboa, Portugal) and colleagues at the Universidad Indoamérica, Ecuador—David Salazar-Valenzuela, Amalia Espinoza-Regalado, and Diego R. Quirola—for conducting and/or assisting with fieldwork and permits in Ecuador; Steve Thurston (AMNH) for assistance with preparing the plates for this contribution; and Ricardo Botero-Trujillo and Stephanie F. Loria for constructive comments on a previous draft of the manuscript. This fieldwork forms part of the Binational Expedition between Colombia and Peru, conducted by SINCHI, to study biodiversity in the Putumayo River basin. Material of T. vachoni was collected under permit MAATE-DBI-CM-2022-0259 issued by the Ministerio del Ambiente, Agua y Transición Ecológica, Ecuador, to David Salazar-Valenzuela. The first author was supported by U.S. National Science Foundation grant DEB 2003382 to the last author.
Copyright © American Museum of Natural History 2024
REFERENCES
Appendices
APPENDIX 1
Material Examined for Phylogenetic Analysis of Troglotayosicus Lourenço, 1981
Material deposited in the following collections: the Instituto de Ciencias Naturales (ICN), Universidad Nacional de Colombia, Bogotá; the American Museum of Natural History (AMNH), New York, including the Ambrose Monell Cryocollection (AMCC); the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia,” National Arachnological Collection (MACN-Ar), Buenos Aires, Argentina; the Museo Javeriano de Historia Natural “Lorenzo Uribe S. J.,” Pontificia Universidad Javeriana (MPUJ), Bogotá, Colombia; the Museo de Zoología (QCAZ), Pontificia Universidad Católica del Ecuador, Quito.
Outgroup
Superstitionia donensis Stahnke, 1940: U.S.A.: California: San Benito County: Griswold Hills, Griswold Creek Canyon New Idria Road, 3.7 mi. S of intersection with Panoche Road, 36°33′29.8″N 120°50′27″W, 380 m, 15.ix.2007, L. Prendini, J. Huff and W.E. Savary, 5 ♂(AMNH); Los Peñasquitos Reserve, Del Mar Mesa, 32°56′21.48″N 117°10′52.8″W, 114 m, 19.ix.2012, L. Prendini and W.E. Savary, 1 ♂, 1 ♀ (AMNH).
Typhlochactas mitchelli Sissom, 1988: MEXICO: Oaxaca: San José Tenango Municipality: Cerro Ocote, 18°08′57″N 96°43′59″W, 5 mi. S of San José de Tenango, iv.1987, A. Grubbs, A. Cressler, and P. Smith, holotype ♂, 1 ♂, 1 subad., ♀ paratypes (AMNH).
Ingroup
Troglotayosicus akaido, sp. nov.: COLOMBIA: Amazonas Department: El Encanto Municipality: Río Caraparaná, Comunidad de San Rafael, 01°41′10.5″S 73°13′18.7″W, 123 m, 23–24. iv.2022, D. Luna and G. Cobette, manual collecting using UV light, inside leaf litter, 30 cm deep, holotype ♂, 2 ♂, 2 ♀, 2 subad. ♀, 1 juv. paratypes (ICN), 2 juv. (AMCC [LP 18087, 18093]).
Troglotayosicus ballvei Botero-Trujillo et al., 2021: ECUADOR: Napo Province: San Pablo de Ushpayacu Canton: Río Hollín, on way to Santo Domingo, 5 km SE of Archidona, Sacha Huagra Lodge, 00°57′19.6″S 77°44′58.7″W, 660 m, 15–16.iii.2014, J.A. Ochoa and R. Botero-Trujillo, manual collecting, holotype ♀ (QCAZ), paratype ♂ (AMNH), 1 juv. (AMCC [LP 12767]).
Troglotayosicus hirsutus Botero-Trujillo and Francke, 2012: COLOMBIA: Nariño Department: Buesaco, 01°22′39″N 77°09′29″W, 1959 m, 2.xii.2011, J.A. Ochoa, leaf litter, 1 subad. ♀, 1 juv. ♀ (AMCC [LP 11284]).
Troglotayosicus humiculum Botero-Trujillo and Francke, 2009: COLOMBIA: Nariño Department: Ricaurte Municipality: Vereda Alto Cartagena, Finca Nueva Estrella, 01°13′15.72″N 77°58′08.58″W, 1617 m, 12.ix.2008, R. Botero-Trujillo, J.P. Botero, and J.A. Ochoa, litter, rainforest, 2 ♀, 1 subad. ♂ (AMNH); 1 subad. ♀ (AMCC [LP 9311]), 3 juv. (AMCC [LP 9312–9314]), 1 ♂, 3 ♀, 1 juv. (ICN); 1 ♂, 3 ♀, 1 juv. (MPUJ).
Troglotayosicus meijdeni Botero-Trujillo et al., 2017: COLOMBIA: Huila Department: Rivera Municipality: Vereda Agua Caliente, area adjacent to Termales Los Ángeles, 02°45′06″N 75°14′17″W, 880 m, i.2014, J.C. González Gómez and J.C. Valenzuela Rojas, holotype ♀(MPUJ-ENT 46833), 2 ♀ paratypes (MACN-Ar); 02°45′24.7″N 75°14′15.9″W, 800 m, i.2017, J.C. González Gómez and J.C. Valenzuela Rojas, paratype ♀ (AMCC [LP 13921]).
Troglotayosicus vachoni Lourenço, 1981: ECUADOR: Morona-Santiago Province: Limón Indanza Canton: Cueva de Los Tayos, Galería de Los Tayos, 03°03′06.1″S 78°12′19.4″W, 447 m, 17–19.xi.2022, J. Blasco-Aróstegui, A. Espinoza-Regalado, and D.R. Quirola, inside cave, under stones and buried in loose soil, collected with UV light, soil sifting with forceps, 1 ♂, 1 ♀ (AMNH), 1 juv. ♂ (AMCC [LP 19028]), 2 juv. ♀ (AMCC [LP 19027]).
APPENDIX 2
Morphological Character Matrix for Phylogenetic Analysis of Troglotayosicus Lourenço, 1981 Character states scored 0–2, ? (unknown) or - (inapplicable). Refer to appendix 3 for character descriptions.

APPENDIX 3
List of 131 Characters for Seven Species of Troglotayosicus Lourenço, 1981, and Two Outgroup Species
Refer to appendix 2 for character matrix. Characters from previous analyses corresponding partially or entirely to those in present matrix as follows: L80 = Lamoral (1980: 441), table 1; S89 = Stockwell (1989: 266), table 6; P00 = Prendini (2000: 5), table 3; S&F01 = Soleglad and Fet (2001: 38), appendix C; S&S01 = Soleglad and Sissom (2001: 74, 75), table 9; S&F03 = Soleglad and Fet (2003a: 66), table 3; P04 = Prendini (2004: 40), table 1; F&P08 = Francke and Prendini (2008: 210), table 4; V&P09 = Vignoli and Prendini (2009: 90), appendix 1; PEA10 = Prendini et al. (2010: 7), table 2; MEA12 = Mattoni et al. (2012: 163), table 2; G&P13 = González-Santillán and Prendini (2013: 64), appendix 1; OEA13 = Ochoa et al. (2013: 10), table 2; SEA14a = Santibáñez-Lopez et al. (2014a: 39), table 2; SEA14b = Santibáñez-López et al. (2014b: 647), table 1; G&P15 = González-Santillán and Prendini (2015: 379), appendix 3; PEA21 = Prendini et al. (2021: 136), appendix 2.
Setation
Pigmentation
2. Carapace, tergites, pedipalps, and metasoma (e.g., pedipalpal and metasomal carinae) [V&P09/1; PEA10/1; SEA14b/0; PEA21/2, 3]: 0, infuscate (pigmented); 1, immaculate (not pigmented).
Chelicerae
3. Fixed finger, median and basal teeth, fusion [V&P09/2; PEA10/2; SEA14b/1; PEA21/6]: 0, fused into bicusp (conjoined on a “trunk”); 1, separate, not fused into bicusp (not conjoined on a “trunk”).
4. Fixed finger, number of teeth [V&P09/3; PEA10/3]: 0, four (subdistal present); 1, three (subdistal absent).
5. Movable finger, dorsal margin, number of subdistal teeth [V&P09/5; PEA10/5; SEA14b/3]: 0, two; 1, none.
Carapace
6. Anteromedian projection (epistome) [F&P08/14; V&P09/6; PEA10/6; G&P13/30; OEA13/1; SEA14b/4; G&P15/75; PEA21/11; modified]: 0, absent; 1, present.
7. Anteromedian longitudinal sulcus [V&P09/7; PEA10/7; MEA12/7; G&P13/3; SEA14a/16; SEA14b/5; G&P15/77; modified]: 0, absent or obsolete; 1, present.
8. Lateral ocelli, PLMa ocellus [S89/21; 25; P00/1; S&F03/102; V&P09/8; PEA10/8; SEA14b/6; PEA21/26; modified]: 0, present; 1, absent.
9. Lateral ocelli, PDMi ocellus [S89/21; 25; P00/1; S&F03/102; V&P09/9; PEA10/9; SEA14b/7; PEA21/28; modified]: 0, present, greatly reduced (less than half the size of PLMa ocellus); 1, present, large (half the size of PLMa ocellus); 2, absent.
10. Lateral ocelli, MLMa ocellus [S89/21; 25; P00/1; S&F03/102; V&P09/10; PEA10/10; SEA14b/8; PEA21/25; modified]: 0, present; 1, absent.
11. Lateral ocelli, ADMi ocellus [S89/21; 25; P00/1; S&F03/102; V&P09/11; PEA10/11; SEA14b/9; PEA21/27; modified]: 0, present, greatly reduced (less than half the size of MLMa ocellus); 1, absent.
12. Lateral eyespot [PEA21/29; modified]: 0, absent; 1, present, smaller than PDMi ocellus; 2, present, similar in size to PDMi ocellus.
13. Median ocelli [S89/24; V&P09/12; PEA10/12; SEA14b/10; PEA21/18]: 0, present; 1, absent.
Pedipalp Chela Sexual Dimorphism
Pedipalp Chela Finger Dentition
15. Chela fingers dentition, median denticle row, oblique primary subrows [P04/7; V&P09/14; PEA10/14; OEA13/16; SEA14b/12; rescored for Troglotayosicus vachoni Lourenço, 1981]: 0, imbricate; 1, not imbricate.
16. Chela movable finger dentition, fourth to seventh retrolateral denticles [S&S01/33; V&P09/17–20; PEA10/17–20; G&P13/98; SEA14b/15; G&P15/143; PEA21/91; modified]: 0, present; 1, absent.
17. Chela movable finger dentition, fifth to seventh prolateral denticles [P04/7; V&P09/21–24; PEA10/21–24; G&P13/99; SEA14b/19; PEA21/93]: 0, present; 1, absent.
18. Chela movable finger dentition, prolateral denticle development [S&F03/54; V&P09/26; PEA10/26; SEA14b/22]: 0, proximal four prolateral denticles significantly larger; 1, all prolateral denticles similar in size.
19. Chela fixed finger, distal diastema (notch) to accommodate terminal denticle of movable finger [S&S01/33; V&P09/27; PEA10/27; SEA14b/24]: 0, weakly developed or absent; 1, present, well developed.
20. Chela fingers, terminus [V&P09/29; PEA10/29; G&P13/96; SEA14b/26]: 0, fixed finger, terminal denticle slightly larger than preceding denticles, fingertips interlocking evenly when closed, movable finger at most slightly displaced retrolaterally; 1, fixed finger, terminal denticle considerably larger than preceding denticles, hooklike, fingertips interlocking unevenly when closed, movable finger markedly displaced retrolaterally.
Pedipalp Chela Ornamentation
21. Chela fixed finger, surfaces, proximal half, texture [V&P09/30; PEA10/30; SEA14b/27]: 0, smooth; 1, dorsal, retrolateral, and prolateral surfaces granular.
22. Chela manus, prodorsal (PD) carina, vertical development [G&P13/53; G&P15/98; PEA21/63]: 0, absent; 1, obsolete (weakly sclerotized, if at all, not protruding above intercarinal surfaces).
23. Chela manus, dorsomarginal (DMA) carina, vertical development [P00/22; S&S01/24; V&P09/34; PEA10/34; G&P13/54; SEA14a/48; SEA14b/31]: 0, absent; 1, obsolete (weakly sclerotized, if at all, not protruding above intercarinal surfaces).
Pedipalp Patella Ornamentation
24. Patella prolateral surface, dorsal process (dorsal “patellar spur”), development [P00/18; S&S01/15–17; S&F03/97, 98; V&P09/42; PEA10/42; Sl14/39; PEA21/51, 52]: 0, well developed projection comprising one or more prominent, spiniform granules; 1, projection absent or very weakly developed, comprising at most a low granule.
25. Patella, prodorsal carina, vertical development [V&P09/47; PEA10/47; SEA14b/44; rescored for Troglotayosicus humiculum Botero-Trujillo & Francke, 2009, and T. vachoni]: 0, obsolete (weakly sclerotized, if at all, not protruding above intercarinal surfaces); 1, distinct (markedly sclerotized, protruding above intercarinal surfaces); 2, absent.
26. Patella, proventral carina, vertical development [V&P09/48; PEA10/48; SEA14b/45; rescored for T. humiculum and T. vachoni]: 0, obsolete (weakly sclerotized, if at all, not protruding above intercarinal surfaces); 1, distinct (markedly sclerotized, protruding above intercarinal surfaces); 2, absent.
Pedipalp Femur Ornamentation
27. Femur, retrodorsal carina, vertical development [V&P09/50; PEA10/50; SEA14b/47; PEA21/47; rescored for T. humiculum and T. vachoni]: 0, obsolete (weakly sclerotized, if at all, not protruding above intercarinal surfaces); 1, distinct (markedly sclerotized, protruding above intercarinal surfaces); 2, absent.
28. Femur, prodorsal carina, vertical development [V&P09/51; PEA10/51; SEA14b/48; PEA21/38; rescored for T. humiculum and T. vachoni]: 0, obsolete (weakly sclerotized, if at all, not protruding above intercarinal surfaces); 1, distinct (markedly sclerotized, protruding above intercarinal surfaces); 2, absent.
29. Femur, retroventral carina, vertical development [V&P09/52; PEA10/52; G&P13/43; SEA14b/49; G&P15/88; rescored T. humiculum and T. vachoni]: 0, obsolete (weakly sclerotized, if at all, not protruding above intercarinal surfaces); 1, distinct (markedly sclerotized, protruding above intercarinal surfaces); 2, absent.
Pedipalp Femur Trichobothria
30. Femur, trichobothrium d1 [V&P09/56; PEA10/56; SEA14b/53]: 0, present, full size; 1, absent.
31. Femur, trichobothrium d2 [V&P09/57; PEA10/57; SEA14b/54]: 0, absent; 1, present, full size.
32. Femur, trichobothrium d3 [V&P09/58; PEA10/58; SEA14b/55; PEA21/42]: 0, absent; 1, present, full size.
33. Femur, trichobothrium d4 [V&P09/59; PEA10/59; SEA14b/56; PEA21/43]: 0, present, full size; 1, absent.
34. Femur, trichobothrium d5 [V&P09/60; PEA10/60; SEA14b/57; PEA21/44]: 0, absent; 1, present, full size.
Pedipalp Patella Trichobothria
35. Patella, trichobothrium d1 [V&P09/61; PEA10/61; SEA14b/58]: 0, present, full size; 1, present, petite.
36. Patella, trichobothrium d2 [V&P09/62; PEA10/62; SEA14b/59]: 0, present, full size; 1, present, petite.
37. Patella, trichobothrium i1 [V&P09/63; PEA10/63; SEA14b/60]: 0, present, full size; 1, absent.
38. Patella, trichobothrium i2 [S&F01/28; V&P09/64; PEA10/64; SEA14b/61; PEA21/53]: 0, absent; 1, present, full size.
39. Patella, trichobothrium v2 [S&F01/47–49; S&F03/47–49; V&P09/65; PEA10/65; SEA14b/62; PEA21/62]: 0, present, full size; 1, absent.
40. Patella, trichobothrium v3 [V&P09/66; PEA10/66; SEA14b/63]: 0, absent; 1, present, full size.
41. Patella, trichobothrium v4 [V&P09/67; PEA10/67; SEA14b/64]: 0, present, full size; 1, absent.
42. Patella, trichobothrium et1 [V&P09/68; PEA10/68; SEA14b/66]: 0, present, full size; 1, absent.
43. Patella, trichobothrium et3 [V&P09/70; PEA10/70; SEA14b/68]: 0, present, full size; 1, present, petite.
44. Patella, trichobothrium et5 [V&P09/72; PEA10/72; SEA14b/70]: 0, present, full size; 1, absent.
45. Patella, trichobothrium et6 [V&P09/73; PEA10/73; SEA14b/71]: 0, absent; 1, present, full size.
46. Patella, trichobothrium et9 [V&P09/76; PEA10/76; SEA14b/74]: 0, absent; 1, present, full size.
47. Patella, trichobothrium em2 [V&P09/78; PEA10/78; SEA14b/76]: 0, absent; 1, present, full size.
48. Patella, trichobothrium esb2 [V&P09/81; PEA10/81; SEA14b/79]: 0, absent; 1, present, petite.
49. Patella, trichobothrium esb3 [V&P09/82; PEA10/82; SEA14b/80]: 0, present, petite; 1, absent.
50. Patella, trichobothrium esb4 [V&P09/83; PEA10/83; SEA14b/81]: 0, absent; 1, present, petite.
51. Patella, trichobothrium eb1 [V&P09/85; PEA10/85; SEA14b/83]: 0, present, full size; 1, absent.
52. Patella, trichobothrium eb3 [V&P09/87; PEA10/87; SEA14b/85]: 0, absent; 1, present, petite; 2, present, full size.
53. Patella, trichobothrium eb4 [V&P09/88; PEA10/88; SEA14b/86]: 0, absent; 1, present, full size.
54. Patella, trichobothrium eb5 [V&P09/89; PEA10/89; SEA14b/87]: 0, absent; 1, present, full size.
55. Patella, trichobothrium eb6 [V&P09/90; PEA10/90; SEA14b/88]: 0, present, full size; 1, absent.
56. Patella, trichobothrium eb7 [V&P09/91; PEA10/91; SEA14b/89]: 0, present, full size; 1, absent.
57. Patella, trichobothrium eb8 [V&P09/92; PEA10/92; SEA14b/90]: 0, present, full size; 1, absent.
58. Patella, trichobothrium eb9 [V&P09/93; PEA10/93; SEA14b/91]: 0, absent; 1, present, full size.
Pedipalp Chela Trichobothria
59. Chela, trichobothrium V1 [V&P09/98; PEA10/98; SEA14b/96; PEA21/85]: 0, present, full size; 1, present, petite.
60. Chela, trichobothrium D1, position: 0, proximal on chela manus, slightly distal to trichobothrium Eb3; 1, submedial on the manus, approximately midway between trichobothria Eb3 and d8.
61. Chela, trichobothrium e8, position relative to trichobothrium m4: 0, proximal to; 1, distal to.
62. Chela, trichobothrium i3, position [SEA14a/65]: 0, closer to base of fixed finger than to trichobothrium i4; 1, midway between base of fixed finger and trichobothrium i4.
63. Chela, trichobothrium V3 [V&P09/100; PEA10/100; SEA14b/98]: 0, present, full size; 1, absent.
64. Chela, trichobothrium V5 [V&P09/102; PEA10/102; SEA14b/100]: 0, present, full size; 1, absent.
65. Chela, trichobothrium V6 [V&P09/103; PEA10/103; SEA14b/101]: 0, absent; 1, present, full size.
66. Chela, trichobothrium D1 [V&P09/106; PEA10/106; SEA14b/104]: 0, present, full size; 1, present, petite.
67. Chela, trichobothrium D2 [V&P09/107; PEA10/107; SEA14b/105]: 0, present, full size; 1, absent.
68. Chela, trichobothrium D3 [V&P09/108; PEA10/108; SEA14b/106]: 0, present, full size; 1, absent.
69. Chela, trichobothrium Et4 [S&F01/21; S&F03/21; V&P09/110; PEA10/110; SEA14b/108; PEA21/79]: 0, present, petite; 1, absent.
70. Chela, trichobothrium Et5 [V&P09/111; PEA10/111; SEA14b/109]: 0, present, full size; 1, absent.
71. Chela, trichobothrium Est1 [S89/86; V&P09/113; PEA10/113; PEA21/76]: 0, absent; 1, present, full size.
72. Chela, trichobothrium Est6 [V&P09/117; PEA10/117; OEA13/32; SEA14b/113]: 0, absent; 1, present, petite.
73. Chela, trichobothrium d5 [V&P09/122; PEA10/122; SEA14b/118]: 0, present, full size; 1, absent.
74. Chela, trichobothrium d7 [V&P09/124; PEA10/124; SEA14b/120]:0, absent; 1, present, full size.
75. Chela, trichobothrium d8 [V&P09/125; PEA10/125; SEA14b/121]: 0, absent; 1, present, petite; 2, present, full size.
76. Chela, trichobothrium m2 [V&P09/127; PEA10/127; SEA14b/123]: 0, absent; 1, present, full size.
77. Chela, trichobothrium m4 [V&P09/129; PEA10/129; SEA14b/125]: 0, absent; 1, present, petite.
78. Chela, trichobothrium e6 [V&P09/133; PEA10/133; SEA14b/129]: 0, absent; 1, present, petite; 2, present, full size.
79. Chela, trichobothrium e7 [V&P09/134; PEA10/134; SEA14b/130]: 0, absent; 1, present, full size.
80. Chela, trichobothrium e8 [V&P09/135; PEA10/135; OEA13/31; SEA14b/131]: 0, present, full size; 1, absent.
81. Patella, trichobothrium V1, position relative to trichobothrium esb1 [V&P09/136; PEA10/136; SEA14b/132]: 0, proximal to; 1, aligned with; 2, distal to.
82. Chela, trichobothrium d4, position relative to trichobothrium e5 [V&P09/137; PEA10/137; SEA14b/133]: 0, distal to; 1, aligned with.
Legs
83. Legs I and II, retrolateral pedal spurs [L80/11; S89/90; P00/63; S&F03/60; V&P09/139; PEA10/139; SEA14b/135; PEA21/99; rescored for T. humiculum]: 0, present; 1, absent.
84. Legs III and IV, retrolateral pedal spurs [L80/11; S89/90; P00/63; S&F03/60; V&P09/140; PEA10/140; rescored for T. humiculum]: 0, present; 1, absent.
85. Legs I–IV basitarsi, rows of brushlike macrosetae [G&P13/125, 126; G&P15/169, 170]: 0, comprising clusters of macrosetae; 1, comprising regular, comblike row of macrosetae; 2, absent.
86. Leg III basitarsus, proventral row of macrosetae: 0, present; 1, absent.
87. Leg IV basitarsus, proventral row of macrosetae: 0, present; 1, absent.
88. Leg IV basitarsus, promedian row of macrosetae: 0, absent; 1, present.
89. Leg IV basitarsus, retrodorsal row of macrosetae: 0, present; 1, absent.
90. Leg I basitarsus, retroventral row of macrosetae: 0, present; 1, absent.
91. Leg III basitarsus, retroventral row of macrosetae: 0, present; 1, absent.
92. Leg IV basitarsus, retroventral row of macrosetae: 0, present; 1, absent.
93. Leg I–IV basitarsi, prolateral and retrolateral surfaces, distal marginal tubercles with macrosetae: 0, present; 1, absent.
94. Leg I–IV telotarsi, ventral macrosetae, arrangement [L80/9; S89/93, 94, 97; P00/68, 70; S&S01/83, 84, 88, 89; S&F03/57, 58; V&P09/154; PEA10/154; SEA14b/144; PEA21/102]: 0, irregularly arranged, not forming distinct rows; 1, regularly arranged into pair of distinct ventrosubmedian rows.
95. Leg I–IV telotarsi, ventral macrosetae, development [L80/9; S89/93, 94, 97; P00/68, 70; S&S01/83, 84, 88, 89; S&F03/57, 58; V&P09/155; PEA10/155; SEA14b/145; rescored for T. vachoni]: 0, setose, clustered; 1, subspiniform, separated.
Sternum
96. Sternum, vertical “compression” [S&S01/70; S&F03/67; V&P09/157; PEA10/157; SEA14b/147]: 0, minimal, length less than posterior width; 1, absent, length greater than or equal to posterior width.
97. Sternum apex, shape [S&F03/69; V&P09/158; PEA10/158; SEA14b/148; rescored for T. humiculum and T. vachoni]: 0, pointed; 1, rounded.
98. Sternum lateral lobes, development [S&F03/69; V&P09/159; PEA10/159; SEA14b/149]: 0, strongly convex (lobes create deep cleft medially); 1, weakly convex (lobes create shallow cleft medially); 2, flat.
Tergites
99. Tergite VII, dorsosubmedian carinae, longitudinal development [V&P09/160; PEA10/160; SEA14b/150]: 0, vestigial (few posterior granules); 1, absent.
100. Tergite VII, dorsolateral carinae, longitudinal development [V&P09/161; PEA10/161; SEA14b/151]: 0, vestigial (few posterior granules); 1, absent.
Pectines
101. Pectinal basal plate, anterior margin shape (♂): 0, concave, with deep anteromedian notch; 1, concave, with shallow anteromedian notch; 2, linear, without anteromedian notch.
102. Pectinal basal plate, anterior margin shape (♀): 0, concave, with deep anteromedian notch; 1, concave, with shallow anteromedian notch; 2, linear, without anteromedian notch.
103. Pectinal fulcra [S&S01/73; S&F03/104; V&P09/163; PEA10/163; SEA14b/152; PEA21/112]: 0, present; 1, absent.
104. Pectinal lamellae, sutures, longitudinal suture between second (subdistal) marginal lamella and second (subdistal) or second and third medial lamellae [V&P09/165; PEA10/165; SEA14b/154; PEA21/108, 109]: 0, present, lamellae separated; 1, absent, lamellae fused.
105. Pectinal lamellae, sutures, transverse suture between second (subdistal) and third medial lamellae [V&P09/166; PEA10/166; SEA14b/155]: 0, present, lamellae separated; 1, absent, lamellae fused.
106. Pectinal lamellae, sutures, transverse suture between fourth and fifth medial lamellae [V&P09/168; PEA10/168; SEA14b/157]: 0, present, lamellae separated; 1, absent, lamellae fused.
107. Pectines, proximal medial lamella (scape), angle (♂) [V&P09/169; PEA10/169; SEA14b/158; PEA21/111; G&P13/161; G&P15/205; rescored for T. humiculum]: 0, obtuse, greater than 90° but less than 180°; 1, approximately 90°; 2, acute, less than 90°.
108. Pectinal length, expressed relative to length of coxa of leg IV (♀) [S&F03/103; V&P09/171; PEA10/171; G&P13/160; G&P15/204; rescored for T. humiculum]: 0, moderate, distal edge reaching to, but not beyond, distal edge of coxa; 1, short, distal edge not reaching to distal edge of coxa.
109. Pectinal teeth, count (♂) [V&P09/172; PEA10/172; SEA14b/159; G&P15/0; PEA21/115]: 0, six; 1, five; 2, seven or more.
110. Pectinal teeth, count (♀) [V&P09/173; PEA10/173; SEA14b/160; G&P15/1; PEA21/116]: 0, six; 1, five; 2, seven.
111. Pectinal teeth, shape [V&P09/174; PEA10/174; SEA14b/161]: 0, curved, slightly overlapping; 1, straight, nonoverlapping.
Sternites
112. Sternite V, posteromedian surface (♂) [V&P09/175; PEA10/175; G&P13/169; G&P15/213]: 0, unmodified; 1, smooth, raised surface.
113. Book lung spiracles, shape [L80/20; S&F03/101; V&P09/177; PEA10/177; OEA13/36; SEA14b/163; PEA21/123]: 0, oval; 1, round.
Metasoma
114. Metasomal segments I–IV, dorsolateral carinae, vertical development [V&P09/178, 180; PEA10/178, 180; SEA14b/164; G&P15/217; PEA21/124; OEA13/38; modified]: 0, distinct (moderately sclerotized, protruding slightly above intercarinal surfaces); 1, distinct (markedly sclerotized, protruding markedly above intercarinal surfaces); 2, obsolete (weakly sclerotized, if at all, not protruding above intercarinal surfaces).
115. Metasomal segments I–III, dorsolateral carinae, posterior granules [V&P09/179; PEA10/179; G&P13/177; SEA14b/165; G&P15/221]: 0, significantly larger than preceding granules; 1, not noticeably larger than preceding granules.
116. Metasomal segment I–IV, lateral supramedian carinae, vertical development: 0, obsolete (weakly sclerotized, if at all, not protruding above intercarinal surfaces); 1, distinct (markedly sclerotized, protruding above intercarinal surfaces); 2, absent.
117. Metasomal segments I–III, median lateral carinae, vertical development [V&P09/181; PEA10/181; G&P13/175; OEA13/39; SEA14b/167; G&P15/219; rescored for S. donensis, T. humiculum, and T. vachoni]: 0, distinct (markedly sclerotized, protruding above intercarinal surfaces); 1, obsolete (weakly sclerotized, if at all, not protruding above intercarinal surfaces); 2, absent.
118. Metasomal segment IV, median lateral carinae, vertical development [V&P09/182; PEA10/182; G&P13/176; OEA13/39; SEA14b/168; G&P15/220]: 0, absent; 1, distinct (markedly sclerotized, protruding above intercarinal surfaces).
119. Metasomal segment V, median lateral carinae, vertical development [S&F03/86; V&P09/183; PEA10/183; G&P13/174; SEA14b/169; G&P15/218; PEA21/128]: 0, obsolete (weakly sclerotized, if at all, not protruding above intercarinal surfaces); 1, distinct (markedly sclerotized, protruding above intercarinal surfaces).
120. Metasomal segments I–III, median lateral carinae, longitudinal development [V&P09/184; PEA10/184; SEA14b/170; PEA21/126]: 0, present, incomplete, restricted to posterior half of segment; 1, present, complete; 2, absent.
121. Metasomal segments II–IV, lateral inframedian carinae, longitudinal development [V&P09/187, 188; PEA10/187, 188; G&P13/184, 188; SEA14b/173, 174]: 0, absent; 1, present, complete; 2, present, incomplete, restricted to posterior half of each segment.
122. Metasomal segments I and II, ventrolateral and ventrosubmedian carinae, texture [V&P09/189, 190; PEA10/189, 190; G&P13/186; SEA14b/175, 176; G&P15/230]: 0, absent (smooth surface); 1, present, sparsely granular.
123. [189, 190] Metasomal segment IV, ventrolateral and ventrosubmedian carinae, longitudinal development [V&P09/189, 190; PEA10/189, 190; G&P13/186; OEA13/40; SEA14b/175, 176; G&P15/230]: 0, absent (smooth surface); 1, incomplete, sparsely granular in anterior half of segment; 2, complete.
124. Metasomal segment V, ventrolateral, ventromedian, and ventrosubmedian carinae, vertical development [V&P09/191, 193; PEA10/191, 193; G&P13/187; SEA14b/177; G&P15/231; rescored for T. humiculum]: 0, distinct (markedly sclerotized, protruding above intercarinal surfaces); 1, absent.
125. Metasomal segment V, ventrolateral and ventrosubmedian carinae, longitudinal development [V&P09/192; PEA10/192; G&P13/188; OEA13/44; SEA14b/178; G&P15/232; rescored for T. humiculum]: 0, restricted to posterior two-thirds of segment; 1, complete.
126. Metasomal segment V, ventromedian carina, longitudinal development [OEA13/45]: 0, complete; 1, restricted to posterior two-thirds of segment.
Telson
127. Telson vesicle, ventrolateral and ventrosubmedian carinae, longitudinal development [PEA21/137]: 0, present, complete; 1, vestigial, reduced to few granules at anteroventral margin of segment; 2, absent.
128. Telson vesicle, lateral inframedian carinae, vertical development: 0, present (sclerotized, protruding above intercarinal surfaces); 1, absent.
129. Telson vesicle, width relative to metasomal segment V, posterior width [V&P09/194; PEA10/194; SEA14b/180]: 0, similar to; 1, broader than.
130. Telson vesicle, anterodorsal lateral condyles [V&P09/195; PEA10/195; SEA14b/181; modified]: 0, small; 1, absent; 2, large.
Ecomorphotype