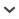This study shows the abundance of contamination by microplastics (MPs) and the first record of contamination by MPs in bats. Additionally, we tried to understand the mechanism of the environmental contamination of bats. Therefore, the digestive and respiratory tracts from 81 adult bats belonging to 25 species were extracted for analysis. Bats were captured in different locations in the Brazilian Amazon (Altamira, Bragança, Brasil Novo, Medicilândia, Nova Timboteua, Placas, São Félix do Xingu, Uruará and Vitória do Xingu, all in the state of Pará). The results showed that all species were contaminated with MPs in at least one of the analyzed systems. For the digestive system, the form of contamination occurs through bioaccumulation and biomagnification by the ingestion of contaminated food or water. In the case of the respiratory system, contamination occurs through the inhalation of MPs suspended in the atmospheric air. The different foraging characteristics of bats, the type of capture strategy for this food, and the type of habitat reinforce the idea that plastic contaminants are present in all environments.
Este estudio muestra la abundancia de contaminación por microplásticos (MP) y el primer registro de contaminación por PM en murciélagos. Además, intentamos comprender el mecanismo de contaminación ambiental de los murciélagos. Por lo tanto, se extrajeron para su análisis los tractos digestivo y respiratorio de 81 murciélagos adultos de 25 especies. Los murciélagos fueron capturados en diferentes localidades de la Amazonía brasileña (Altamira, Bragança, Brasil Novo, Medicilândia, Nova Timboteua, Placas, São Félix do Xingu, Uruará y Vitória do Xingu, todos en el estado de Pará). Los resultados mostraron que todas las especies estaban contaminadas con PM en al menos uno de los sistemas analizados. Para el sistema digestivo, la forma de contaminación se produce por bioacumulación y biomagnificación por la ingestión de alimentos o agua contaminados. Mientras que para el sistema respiratorio, la contaminación se produce por la inhalación de PM suspendidas en el aire atmosférico. Las diferentes características de alimentación de los murciélagos, el tipo de estrategia de captura de este alimento y el tipo de hábitat refuerzan la idea de que los contaminantes plásticos están presentes en todos los entornos.
Introduction
Plastics are polymers derived from petroleum and of anthropogenic origin, considered contaminants of emerging concern, and have gained global attention due to their abundance, durability, and persistence (Thompson et al., 2009; Anderson et al., 2016; Wilson et al., 2021). The properties of plastics, such as low cost and versatility, make their use widespread in society, being used in the domestic, automotive, and textile industries until they become a serious environmental problem due to improper disposal (Napper and Thompson, 2020). Plastic that is no longer useful enters the environment as plastic waste that can be divided into macroplastics (MCPs) and microplastics (MPs) (Steensgaard et al., 2017). Macroplastics comprise macro (> 25 cm) and mesoplastics (5 mm to 25 cm) sizes, while microplastics comprise micro (1 to 5 mm) and nano (< 1 mm) sizes (Wagner et al., 2014). MPs can be of primary origin, produced in micro size, or secondary due to the degradation product of larger pieces (Wright et al., 2013; Boucher and Friot, 2017). In terms of shape, particles can be spherical, like pellets, have irregular shapes like fragments and films, or elongated and thin like fibers (McCormick et al., 2016). Plastics have already been found in fish (Ribeiro-Brasil et al., 2020), birds (Tokunaga et al., 2023), bottled water (Li et al., 2023) and human breast milk (Ragusa et al., 2022). In the Americas, studies on this topic are still scarce (Ayala et al., 2023).
The degradation of plastic textile fibers produces, for example, microplastics called microfibers; this degradation product has been observed in atmospheric precipitation, becoming breathable microfibers (Gasperi et al., 2018). This fact suggests potential exposure of microfibers to organisms that present pulmonary respiration, such as humans and bats (Zhang et al., 2020). Microfibers can also be deposited on the surfaces of fruits and terrestrial organisms (Rillig et al., 2017). In this way, bats can absorb food and inhale aerosolized microfibers, allowing contamination by ingestion and inhalation, respectively.
MPs are the plastic waste most commonly found in the environment and of the most significant concern, as they are considered the easiest to spread and the most assimilated by organisms (Dris et al., 2016; Duis and Coors, 2016; Horton et al., 2017; Windsor et al., 2019; Dahms et al., 2020; He et al., 2020; Miller et al., 2020; Akhbarizadeh et al., 2021; Baho et al., 2021; Kumari et al., 2022). The effects of MP absorption are not yet fully elucidated, but there is already knowledge about ingestion by various organisms, ranging from aquatic (Ribeiro-Brasil et al., 2020; Jawad et al., 2021) to terrestrial environments (Lahive et al., 2019).
Bats exhibit a significant morphological and behavioral diversification, feeding on fruits, nectar, blood, insects, and vertebrates (Kalko, 1998). They play critical ecological roles in the ecosystem, such as pollination, seed dispersal, and insect control, including agricultural pest control (Fenton et al., 1999; Estrada and Coates-Estrada, 2002; Castro-Luna and Galindo-González, 2012; Kasso and Balakrishnan, 2013; Rodríguez-San Pedro et al., 2020; Aguiar et al., 2021; de Jong et al., 2021; Suripto, 2021). In addition, some species of pioneer plants, necessary for regeneration and ecological succession of degraded areas, are pollinated and dispersed exclusively by bats (Passos and Passamani, 2003). Because of the important services they provide, bats are considered key species in tropical forests (Fleming and Heithaus, 1981).
Studies on the ingestion or inhalation of microplastics by bats do not exist, and in situ observations of MPs contamination have only been reported in other organisms, such as marine organisms (Miller et al., 2020), land plants (Kumari et al., 2022), and freshwater fish (Ribeiro-Brasil et al., 2022). Plastic residues have already been found in fish from farms in Rondônia (Dantas Filho et al., 2023), and in other streams in the municipalities of Pará, such as Barcarena, Ipixuna do Pará, Concórdia do Pará, and Tomé-Açu (Ribeiro-Brasil et al., 2020), and in the Xingu River (Andrade et al., 2019) that passes through the city of Altamira. Moreover, the contamination of MPs in human organs has been demonstrated. This contamination is possibly through ingestion and inhalation; it is likely that bats are absorbing plastic waste from the environment, either directly, by inhaling particles from the air (Pauly et al., 1998; Gasperi et al., 2018), or indirectly, by ingesting contaminated food (Gross, 2015; da Costa Araújo and Malafaia, 2021). Bats, like humans, because both have similar respiratory systems, may be susceptible to similar contamination. Thus, the objective of this study was to identify how the ingestion and inhalation of microplastics by bats in the eastern Brazilian Amazon occurs and to confirm the presence of plastic waste through visual analysis and inspection of materials collected from bats.
Materials and Methods
Sample Collection and Species Studied
The samplings were carried out at 26 points, between 2017 and 2021, four urban points and 22 rural point, all located in the following municipalities: Altamira, Brasil Novo, Placas, Nova Timboteua, Bragança, São Félix do Xingu, Uruará, Vitória do Xingu and Medicilândia, all located in the state of Pará (Fig. 1 and Table 1). Some rural points were carried out in cocoa plantations and/or natural vegetation, the urban points were collected within cities or in nearby places. The region has a tropical climate of type Am, according to the Köppen climate classification (Peel et al., 2007), with an average temperature of 26.1°C and an average annual rainfall of 2,000 millimeters.
Bats were sampled using ten mist nets (9 × 2.5 m), open at sunset and remaining for six hours, inspected every half hour. The bats were placed in 100% cotton fabric bags and taken to the Laboratory of Ecology of Altamira ― LABECO at the Federal University of Pará, Altamira campus. Afterwards, collected individuals were euthanized by cervical dislocation, and morphometric data (total length, foot length, ear length, tragus length, forearm length, and body mass) were measured. Subsequently, bats were fixed with 10% formaldehyde and stored in glass jars with 70% alcohol in the ChiroXingu Bat Collection: Center for studies in ecology and conservation of bats. The ChiroXingu research group collected the bats from April to August 2017, September and October 2018, January to June 2020 and July 2021. Each sampling, transport and preservation of the sampled specimens were carried out in accordance with the relevant guidelines and regulations of the Sistema de Autorizacão e Informacão em Biodiversidade, Instituto Chico Mendes de Conservacão da Biodiversidade, Ministerio do Meio Ambiente (license No. 57294-2 granted by the last author). All methods were performed according to ARRIVE guidelines as study design, sample size, statistical methods, and experimental animals. However, the protocols referring to the following works were also followed to avoid contamination of the sample in the laboratory (Nuelle et al., 2014; Wagner et al., 2014; Devriese et al., 2015; Ribeiro-Brasil et al., 2020).
Fig. 1.
Geographical location of the collection points where bats were sampled from 2017 to 2021 by ChiroXingu: Center for Studies in Ecology and Conservation of Bats

Table 1.
Geographical coordinates of the points and locations where bats were sampled during the years 2017 to 2021 by ChiroXingu

Material Analysis
Extraction of the biological tissues
Organs of the digestive and respiratory systems were removed completely from each fluid-preserved specimen. The digestive system was removed from esophagus to anus, and respiratory system from the trachea to lungs. The entire process was carried out inside a laminar flow hood to avoid contamination of the samples.
Digestion of the biological tissues
The samples were put into sanitized glass vials containing potassium hydroxide (KOH; 10%, V/V) to dissolve the tissues (Ghosal et al., 2018). They were placed in an oven with a temperature of 60°C for seven days with modified protocol by (Lavoy and Crossman, 2021) to accelerate the sample digestion process. After tissue digestion, samples were filtered through a 0.2 µm porosity membrane with a vacuum pump. Membranes were stored in Petri dishes, protected by aluminum foil envelopes, and returned to the oven for 24 h at 60°C for drying the membranes. Aluminum was used to avoid contamination of samples in the work environment.
Visual analysis of plastic waste
The samples were analyzed under a stereo microscope with a magnification of 100 times (Digilab-microscope Stereo Trinocular DI-106T zoom). The membranes were scanned from left to right, top to bottom. Each item was photographed and identified and placed into two categories: microplastics (MPs) length range from 1 to 5 mm) and mesoplastics (MSPs) range from 5 mm to 25 cm — Wagner et al., 2014).
Quality assurance and quality control (QA/QC)
For material classification, the following criteria were followed: a) residues considered as fibers that had a structure like animal joints were disregarded; b) only plastic waste that had the same pattern from one end to the other was considered; c) confirmation of plastic for smaller particles was done through the hot needle test (Devriese et al., 2015). The hot needle test is placing the hot needle over the sample, if the sample changes shape or shrinks, it is because this sample is considered plastic.
All the necessary precautions were taken for laboratory procedures, such as wearing clothes and gowns made of only 100% cotton. All analysis material was previously washed with distilled water and filtered before use. The membranes used in the filtration process were covered with aluminum foil. In addition, we followed an analytical approach for monitoring microplastics in marine sediments where plastics were counted and removed from the samples collected from bats (we counted only the plastic residues that came from the bats, not sediment or other environmental substrates — Nuelle et al., 2014).
Statistical Analysis
A t-test for separate variances was performed to compare the amount of plastic waste between respiratory and digestive systems. The analysis was done using the software provided by R Core Team (2021).
Results
We analyzed 81 individuals from 25 species and three families; the most abundant species was Carollia perspicillata with nine individuals, while the rarest species, with a single record, were: Saccopteryx bilineata, Lophostoma carrikeri, Phyllostomus elongatus and Artibeus gnomus (Table 2). Seventy-eight individuals (96.3%) were contaminated by plastic residues in at least one of the analyzed organs (lung, stomach, and/or intestine).
One hundred fifty-eight samples were analyzed, representing 77 respiratory and 81 digestive systems (Table 2). There was a significant difference between the systems (t = 4.33, d.f. = 98.6, P < 0.001), with the digestive system being more affected (0, SD: 4.59, 5.62) than the respiratory system (1.73, 1.90 — Fig. 2). Only the respiratory system of Pteronotus gymnonotus, A. gnomus and Sturnira giannae and the digestive system of L. carrikeri were not contaminated by plastic residues (Table 2). All plastic waste found in bats was of the fiber type (Fig. 3).
Discussion
MPs' Paths
This study is the first report of contamination by microplastics (MPs) in bats and expands the list of organisms capable of absorbing MPs. In this way, we confirm that MPs can contaminate bats, and this contamination can be through the airways and digestive tracts. Only fiber-type MPs were found in analyzed organs and investigated systems. The digestive system showed higher contamination. The ingestion and/or inhalation of plastic waste, whether through the digestive or respiratory route, are two possibilities in which bats, and other taxonomic groups, including humans, are exposed (Pauly et al., 1998; Galloway, 2015; Ragusa et al., 2021).
The forms of contamination by plastic waste can be through the atmospheric air or the ingestion of contaminated food or water (Revel et al., 2018). The presence of many fibers can be explained by the fact that they are the lightest and most easily dispersed (Covernton et al., 2019). Some studies pointed out that plastic debris is commonly found in the oceanic food web and more recently in the terrestrial food web (He et al., 2020; Miller et al., 2020; Baho et al., 2021; Kumari et al., 2022) with wind appearing to be the primary disperser of plastic, catalyzed by rain (Dris et al., 2016; Akhbarizadeh et al., 2021). In terrestrial environments, the main pathway od exposure is atmospheric fallout (Evangeliou et al., 2020) and different agricultural practices, for example, plastic mulching (Büks and Kaupenjohann, 2020; Crossman et al., 2020; Baho et al., 2021).
Fiber-type microplastics are the most abundant in the environment and come mainly from clothes. Fibers are released into the environment when pieces are washed or when they come loose from wear (Hernandez et al., 2017; Liu et al., 2019). Another vital factor in microfiber contamination is face masks and wet wipes, which have seen a considerable increase in consumption and improper disposal during the COVID-19 pandemic (Fadare and Okoffo, 2020; Shruti et al., 2021). Due to its shape, fibers tend to be retained in the lungs and digestive tract. When inhaled and ingested, the fiber-type plastic waste is the easiest to release by the digestive system (Suran, 2018; Saborowski et al., 2019).
Fig. 2.
Boxplot of t-test showing the difference in the concentration of plastic waste between respiratory and digestive systems

Table 2.
Bat species collected in the Brazilian Amazon, showing the number of micro- and mesoplastic particles recorded for each species. The total (∑) is followed by the mean (0) ± standard deviation (SD). N ― total number of specimens in the study, N* ― number of analyzed systems

Plants can intercept MPs carried by winds and rains, where rough surfaces, such as stems, leaves, flowers, and fruits, can absorb microplastics (estimated 0.13 trillion MPs/cm2) (Liu et al., 2020) and internalize them (Yin et al., 2021). Thus, frugivorous species, such as bats of the subfamilies Carollinae, Rhinophyllinae, and Stenodermatinae, feed on fruits that may be contaminated (Fenton et al., 1999). For example, bats will consume particles that have become trapped in floral exudates and particles that have been internalized. Contamination of the digestive system of fruit bats by MPs suggests bioaccumulation.
The accumulation of plastic contaminants in secondary consumers and at levels above these are well documented in the literature (Dris et al., 2016; Hocking et al., 2017; Horton et al., 2017; de Souza Machado et al., 2018; He et al., 2020; Miller et al., 2020; Kumari et al., 2022), especially for marine environments. For aquatic food chains, evidence of the accumulation of plastic waste was observed in producers, secondary consumers, and even quaternary consumers, thus showing the accumulation and transfer of these contaminants along at least five trophic levels in the marine food chain (Miller et al., 2020).
Despite the growing number of publications in terrestrial environments, no studies described the path of plastic waste from primary producers to tertiary or quaternary consumers (He et al., 2020b). However, the transport of these plastic wastes by the vascular systems of plants has already been reported (Crossman et al., 2020; Li et al., 2020), leading to the presence and consequent accumulation of these residues in roots, leaves, seeds, and fruits (Dietz and Herth, 2011; Kumari et al., 2022). This plastic waste can be primarily from the atmospheric air (Truong et al., 2021), which is subsequently carried to the soil and bodies of water (Dris et al., 2016; Truong et al., 2021). Soil contamination, and consequent contamination of plant vascular systems, can be intensified by the deposition of plastic waste in river waters and areas bordering water bodies. It is estimated that large rivers can be abundant sources of plastic waste, given the urbanization around rivers, in addition to the dendritic and accumulative nature of the drainage basins (Mani et al., 2015; de Souza Machado et al., 2018).
Fig. 3.
Microplastics found in the respiratory and digestive systems of bats. Images A and B refer to mesoplastic fibers. Images C, D, E, and F are microplastic-sized fibers

The transfer of plastic waste between different trophic levels may explain the contamination of insectivorous and omnivorous bats and suggests biomagnification of MPs (Horton et al., 2017; Lusher et al., 2017; de Souza Machado et al., 2018; Miller et al., 2020; Kumari et al., 2022). Biomagnification has been observed for aquatic and terrestrial trophic chains, including small and medium-sized vertebrates and invertebrates, such as annelids and arthropods (Horton et al., 2017; de Souza Machado et al., 2018). Prey contamination can occur in the same way as for plants, through the deposition of residues present in ambient air on the surface of the body. Another route of prey contamination is direct or indirect ingestion of plastic (Al-Jaibachi et al., 2018; Windsor et al., 2019b; Immerschitt and Martens, 2020). In this way, when preyed upon by bats, they contaminate bats (Dris et al., 2016; Horton et al., 2017; de Souza Machado et al., 2018; Kumari et al., 2022).
MPs have already been found in insects of some orders such as Coleoptera and Diptera (Heinlaan et al., 2020) in addition to Ephemeroptera and Trichoptera (Ziccardi et al., 2016; Lusher et al., 2017) all of which are considered food resources by insectivorous bats. In this case, MPs contamination in insects can be either by the accumulation of plastic residues in their exoskeletons or their external structure (Ehlers et al., 2020) since insects have a body surface with bristles that can serve as a substrate for adhesion of MPs. Thus, contamination of terrestrial vertebrates, such as birds (Boucher and Friot, 2017) and bats, occurs through inhalation of air contaminated by MPs, consumption of contaminated water (Carlin et al., 2020), and bioaccumulation and biomagnification through interactions with the environment and food webs (Waite et al., 2018).
Contamination of Respiratory and Digestive Systems
Contamination of the entire respiratory system of bats, demonstrated in our study, is consistent with findings in the literature that the atmospheric air is contaminated in all environments, whether urban, rural, or even those considered pristine (Dris et al., 2016; He et al., 2020; Akhbarizadeh et al., 2021; Truong et al., 2021). Plastic debris present in atmospheric air is considered the initial and primary source of contamination of all other systems (continental or oceanic) (Dris et al., 2016; Akhbarizadeh et al., 2021) and direct inhalation (during breathing) of atmospheric air with plastic debris (Sridharan et al., 2021) is a simple and consistent mechanism for bat respiratory contamination.
Members of the subfamily Phyllostominae in our sample include species of the genera Lophostoma, Phyllostomus, and Tonatia captured in our study, are considered indicators of preserved habitats with primary or secondary vegetation in an advanced state of regeneration (Faria, 2006; Oliveira and Aguiar, 2015; Palheta et al., 2020; Vieira et al., 2021; Weier et al., 2021). This corroborates that plastic waste tends to be a contaminant of emerging concern and is distributed in all environments, either in atmospheric air or in food of bats through consumption of fruits, insects and small vertebrates. Two other examples include insectivorous bats occurring in gaps and edges of vegetation, such as members of Embalonuridae and Mormoopidae that forage in the forest, usually between the canopy and sub-canopy. Additionally, gleaner insectivores, such as Phyllostominae bats that use the interior of the vegetation to forage use sit-and-wait strategy, were found with respiratory and digestive systems contaminated with plastic debris.
Contamination by MPs in terrestrial vertebrates has already been observed in other groups and with concentrations similar to that in bats. A high frequency of MPs (94.1% of individuals) was reported in birds (Zhao et al., 2016). Equal concentration to the frequency of occurrence of MPs observed in the analyzed bats (96.3%). However, the level of contamination of individuals can vary depending on the habitat and behavior of species. In studies by Carlin et al. (2020), where they evaluated the ingestion of MPs in eight species of birds of prey and noted that 100% of the birds were contaminated with MPs in the digestive system. Other studies show less contamination (less than 85%) in stomach samples from two species of bird residing in coastal swamps in Mississippi, USA (Weitzel et al., 2021). In both studies, the dominance of fiber-type MPs was recorded, in agreement with what we observed in our study.
Unlike other authors, we analyzed both the digestive system (from esophagus to anus), with 98.8% MP contamination, and the respiratory system (from trachea to lungs), with 96.1% MP contamination. We obtained a mean (± SD) food intake of 14.9 ± 17.37 MPs and air intake of 5.2 ± 7.17 MPs. Generally, the previous works analyzed only one system or organ, usually stomach or intestines. Analyzing only one system or organ limits the comparison of contamination among flying terrestrial organisms.
In general, studies of MP contamination for terrestrial organisms are scarce compared to aquatic environments. MPs have already been detected in human lungs (Pauly et al., 1998); and 19.5% of samples contained fibers (Jenner et al., 2022). However, other studies show that MP contamination varies from 24% (Chen et al., 2022) to 84.6% (Jenner et al., 2022), depending on the habits and location where the person lives.
Bats showed a higher average lung contamination than humans. However, this result is likely to be false, as the whole lungs were analyzed in bats, unlike in humans, where only a small portion of the tissue was used. In addition, data available in the literature does not allow us to make a better comparison of contaminated tissue area of human lungs with the bat lungs. This difference in the concentration of inhaled plastic particles is possibly due to the high exposure in urban centers where people live, with direct contamination inside homes, on the street, and in the workplace. In addition, other factors, such as lifespan, must be taken into account. Humans are expected to live longer (expectation 70 years) than bats (expectation 40 years) (Podlutsky et al., 2005), and exposure increases over time. Due to longer life expectancy, humans are expected to inhale more plastic particles than bats. It is essential to point out that in both bats and humans, there is a lack of knowledge about the adverse effects these particles leave on the respiratory system.
Consequences of Contamination
The ingestion of MPs is not directly linked to the risk of rapid death and survival of species, but the ingestion influences, over time, ontogenetic development and fitness. Ingested MPs will cause adverse effects, such as altered endocrine functions, decreased pup body mass, and tissue inflammation (Roman et al., 2019). In birds, the ingestion of MPs causes a deceleration in sexual development (Roman et al., 2019), evidenced by the transfer of MPs between adults and nestling (Carey, 2011). MPs can cause a false sense of satiety in high concentrations, leading individuals to starvation (Carbery et al., 2018; Fossi et al., 2018). In addition, microorganisms (Amaral-Zettler et al., 2020; Chang et al., 2022; Wang et al., 2022) and metals (Kutralam-Muniasamy et al., 2021; Zong et al., 2021) may adhere to the surfaces of MPs and be additional contaminates (Kutralam-Muniasamy et al., 2021). Ingestion and inhalation of MPs can cause adverse effects on bat species, including local extinction of species, which can affect ecosystem functions, such as pollination, seed dispersal, and insect control performed by bats.
All bats analyzed show plastic debris in the respiratory and digestive systems. The different foraging characteristics of bats, considering both the type of food (flowers, fruits, invertebrates, and vertebrates) and capture strategy (open areas, clearings, edges of vegetation) and habitat (urban or pristine), reinforce the idea that plastic contaminants are present in all environments (especially the terrestrial environment). We also support the need to analyze various organs/tissues, mainly when referring to the direct routes of contamination (respiratory and digestive), to estimate MP contamination and determine possible sources of contamination. The effect of the environment, type of foraging, and even the kind of food on the accumulation of plastic waste in bats remain to be understood. Thus, research is still needed to identify differences in level of contamination by plastic waste in bats and its relationship with the type of environment, foraging strategy, and food consumed. In addition, analysis of feces of terrestrial organisms makes it possible to make inferences about how much of these ingested MPs are being eliminated from the organism in question.
Acknowledgements
This research benefited from resources from Vale SA's environmental compensation administered by the Centro Nacional de Pesquisa e Conservação de Cavernas (Cecav/ICMBio) and services to the Brazilian Society for the Study of Chiropterans — SBEQ, as part of the DD Program — The Species More Unknown in Brazil and with resources from the Termo de Compromisso de Compensação Espeleológica — TCCE VALE 1/2018 — Edital Ferruginosas 01/2021 under the administration of the Instituto Brasileiro de Desenvolvimento e Sustentabilidade — IABS — IABS.
© Museum and Institute of Zoology PAS
Author Contribution Statement
LLC: collection and/or assembly of data, data analysis and interpretation, and writing the article; DRGR-B: data analysis and interpretation; MGG: critical revision of the article; DMS: data analysis and interpretation, and critical revision of the article; ABAS: collection and/or assembly of data; TBV: research concept and design, collection and/or assembly of data, critical revision and final approval of the article.